Photocrosslinkable Biomaterials for 3D Bioprinting: Mechanisms, Recent Advances, and Future Prospects
- PMID: 39684279
- PMCID: PMC11641133
- DOI: 10.3390/ijms252312567
Photocrosslinkable Biomaterials for 3D Bioprinting: Mechanisms, Recent Advances, and Future Prospects
Abstract
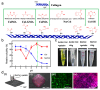
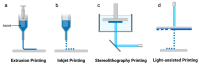
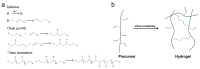
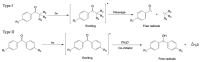
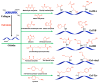
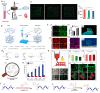
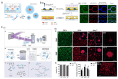
Constructing scaffolds with the desired structures and functions is one of the main goals of tissue engineering. Three-dimensional (3D) bioprinting is a promising technology that enables the personalized fabrication of devices with regulated biological and mechanical characteristics similar to natural tissues/organs. To date, 3D bioprinting has been widely explored for biomedical applications like tissue engineering, drug delivery, drug screening, and in vitro disease model construction. Among different bioinks, photocrosslinkable bioinks have emerged as a powerful choice for the advanced fabrication of 3D devices, with fast crosslinking speed, high resolution, and great print fidelity. The photocrosslinkable biomaterials used for light-based 3D printing play a pivotal role in the fabrication of functional constructs. Herein, this review outlines the general 3D bioprinting approaches related to photocrosslinkable biomaterials, including extrusion-based printing, inkjet printing, stereolithography printing, and laser-assisted printing. Further, the mechanisms, advantages, and limitations of photopolymerization and photoinitiators are discussed. Next, recent advances in natural and synthetic photocrosslinkable biomaterials used for 3D bioprinting are highlighted. Finally, the challenges and future perspectives of photocrosslinkable bioinks and bioprinting approaches are envisaged.
Keywords: 3D bioprinting; hydrogel; photocrosslinkable biomaterials.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



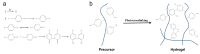




Similar articles
-
Embedded bioprinting for designer 3D tissue constructs with complex structural organization.Acta Biomater. 2022 Mar 1;140:1-22. doi: 10.1016/j.actbio.2021.11.048. Epub 2021 Dec 5. Acta Biomater. 2022. PMID: 34875360 Review.
-
Advances in tissue engineering of vasculature through three-dimensional bioprinting.Dev Dyn. 2021 Dec;250(12):1717-1738. doi: 10.1002/dvdy.385. Epub 2021 Jul 2. Dev Dyn. 2021. PMID: 34115420 Review.
-
Advancing bioinks for 3D bioprinting using reactive fillers: A review.Acta Biomater. 2020 Sep 1;113:1-22. doi: 10.1016/j.actbio.2020.06.040. Epub 2020 Jul 2. Acta Biomater. 2020. PMID: 32622053 Review.
-
Three-Dimensional Bioprinting in Vascular Tissue Engineering and Tissue Vascularization of Cardiovascular Diseases.Tissue Eng Part B Rev. 2024 Jun;30(3):340-358. doi: 10.1089/ten.TEB.2023.0175. Epub 2024 Jan 5. Tissue Eng Part B Rev. 2024. PMID: 37885200 Review.
-
3D printing of functional biomaterials for tissue engineering.Curr Opin Biotechnol. 2016 Aug;40:103-112. doi: 10.1016/j.copbio.2016.03.014. Epub 2016 Apr 1. Curr Opin Biotechnol. 2016. PMID: 27043763 Review.
Cited by
-
Light-activated decellularized extracellular matrix-based bioinks for enhanced mechanical integrity.Mater Today Bio. 2025 May 12;32:101859. doi: 10.1016/j.mtbio.2025.101859. eCollection 2025 Jun. Mater Today Bio. 2025. PMID: 40487170 Free PMC article. Review.
-
Synthesis and Characterization of Photocurable Difunctional Monomers for Medical Applications.Polymers (Basel). 2024 Dec 21;16(24):3584. doi: 10.3390/polym16243584. Polymers (Basel). 2024. PMID: 39771436 Free PMC article.
-
Bioprinting and Intellectual Property: Challenges, Opportunities, and the Road Ahead.Bioengineering (Basel). 2025 Jan 15;12(1):76. doi: 10.3390/bioengineering12010076. Bioengineering (Basel). 2025. PMID: 39851350 Free PMC article. Review.
References
-
- Masri S., Zawani M., Zulkiflee I., Salleh A., Fadilah N.I.M., Maarof M., Wen A.P.Y., Duman F., Tabata Y., Abd Aziz I., et al. Cellular Interaction of Human Skin Cells towards Natural Bioink via 3D-Bioprinting Technologies for Chronic Wound: A Comprehensive Review. Int. J. Mol. Sci. 2022;23:476. doi: 10.3390/ijms23010476. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

