Rhamnolipid-Enriched PA3 Fraction from Pseudomonas aeruginosa SWUC02 Primes Chili Plant Defense Against Anthracnose
- PMID: 39684305
- PMCID: PMC11641530
- DOI: 10.3390/ijms252312593
Rhamnolipid-Enriched PA3 Fraction from Pseudomonas aeruginosa SWUC02 Primes Chili Plant Defense Against Anthracnose
Abstract
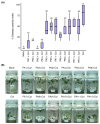
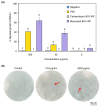
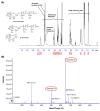
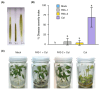
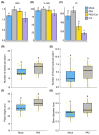
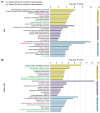
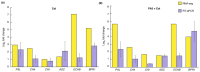
Chili anthracnose, caused by Colletotrichum truncatum, causes significant yield loss in chili production. In this study, we investigated the elicitor properties of a rhamnolipid (RL)-enriched PA3 fraction derived from Pseudomonas aeruginosa SWUC02 in inducing systemic resistance in yellow chili seedlings and antifungal activity against C. truncatum CFPL01 (Col). Fractionation of the ethyl acetate extract yielded 12 fractions, with PA3 demonstrating the most effective disease suppression, reducing the disease severity index to 4 ± 7.35% at 7 days post-inoculation compared with Col inoculation alone (83 ± 23.57%). PA3 also exhibited direct antifungal activity, inhibiting Col mycelial growth by 41 ± 0.96% at 200 µg/mL. Subfractionation revealed PA3 as a mixture of mono- and di-RLs, confirmed by 1H nuclear magnetic resonance and electrospray ionization mass spectrometry data. Additionally, PA3 enhanced seed germination and promoted plant growth without causing phytotoxicity. Transcriptomics revealed that PA3 pre-treatment prior to Col infection primed the defense response, upregulating defense-related genes involved in the phenylpropanoid, flavonoid, and jasmonic acid biosynthesis pathways, as well as those associated with cell wall reinforcement. Our findings highlight the potential of RL-enriched PA3 as both an antifungal agent and a plant defense elicitor, with transcriptome data providing new insights into defense priming and resistance pathways in chili, offering an eco-friendly solution for sustainable anthracnose management.
Keywords: Colletotrichum; Pseudomonas; anthracnose; biocontrol; chili plant; elicitor; induced systemic resistance; rhamnolipid; sustainable agriculture; transcriptome.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Karim K.M.R., Rafii M.Y., Misran A.B., Ismail M.F.B., Harun A.R., Khan M.M.H., Chowdhury M.F.N. Current and prospective strategies in the varietal improvement of chilli (Capsicum annuum L.) specially heterosis breeding. Agronomy. 2021;11:2217. doi: 10.3390/agronomy11112217. - DOI
-
- del Carmen Orozco-Mosqueda M., Fadiji A.E., Babalola O.O., Santoyo G. Bacterial elicitors of the plant immune system: An overview and the way forward. Plant Stress. 2023;7:100138. doi: 10.1016/j.stress.2023.100138. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

