Docosahexaenoic Acid (DHA) Supplementation in a Triglyceride Form Prevents from Polyglutamine-Induced Dysfunctions in Caenorhabditis elegans
- PMID: 39684306
- PMCID: PMC11640917
- DOI: 10.3390/ijms252312594
Docosahexaenoic Acid (DHA) Supplementation in a Triglyceride Form Prevents from Polyglutamine-Induced Dysfunctions in Caenorhabditis elegans
Abstract
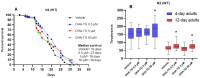
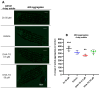
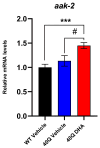
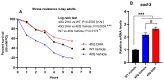
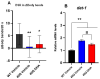
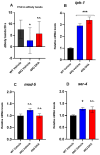
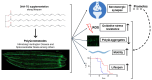
A common hallmark of neurodegenerative diseases is the accumulation of polypeptide aggregates in neurons. Despite the primary cause of these diseases being inherently genetic, their development can be delayed with proper preventive treatments. Long-chain polyunsaturated fatty acids (ω-3 LCPUFA) are promising bioactive nutrients that are beneficial for brain health. In this study, the impact of an oil rich in a structured form of docosahexaenoic acid (DHA) triglyceride (TG) was assessed in a Caenorhabditis elegans model expressing long poly-glutamine (polyQ) chains, which mimics the symptomatology of polyQ-related neurodegenerative diseases such as Huntington's disease (HD), among others. The lifespan, the motility, the number of polyQ aggregates, the oxidative stress resistance, and the cognitive performance associated with sensitive stimuli was measured in mutant nematodes with polyQ aggregates. Overall, DHA-TG at 0.5 µM improved the lifespan, the motility, the oxidative stress resistance, and the cognitive performance of the nematodes, emphasizing the protection against serotonergic synapse dysfunction. Furthermore, the treatment reduced the polyQ aggregates in the nematodes. The data described herein shed light on the connection between DHA and the cognitive performance in neurodegenerative diseases and demonstrated the potential of DHA-TG as nutritional co-adjuvant to prevent the development of polyQ-associated dysfunctions.
Keywords: C. elegans; DHA; Huntington’s; omega-3; polyQ; serotonin.
Conflict of interest statement
Author Ignasi Mora was employed by the company Brudy Technology S.L. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Daldin M., Fodale V., Cariulo C., Azzollini L., Verani M., Martufi P., Spiezia M.C., Deguire S.M., Cherubini M., MacDonald D., et al. Polyglutamine Expansion Affects Huntingtin Conformation in Multiple Huntington’s Disease Models. Sci. Rep. 2017;7:5070. doi: 10.1038/s41598-017-05336-7. - DOI - PMC - PubMed
-
- Trujillo-Del Río C., Tortajada-Pérez J., Gómez-Escribano A.P., Casterá F., Peiró C., Millán J.M., Herrero M.J., Vázquez-Manrique R.P. Metformin to Treat Huntington Disease: A Pleiotropic Drug against a Multi-System Disorder. Mech. Ageing Dev. 2022;204:111670. doi: 10.1016/j.mad.2022.111670. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

