Transcriptome Analysis of Fibroblasts in Hypoxia-Induced Vascular Remodeling: Functional Roles of CD26/DPP4
- PMID: 39684311
- PMCID: PMC11640941
- DOI: 10.3390/ijms252312599
Transcriptome Analysis of Fibroblasts in Hypoxia-Induced Vascular Remodeling: Functional Roles of CD26/DPP4
Abstract
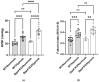
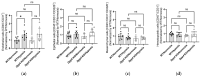
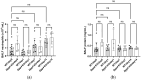
In hypoxic pulmonary hypertension (PH), pulmonary vascular remodeling is characterized by the emergence of activated adventitial fibroblasts, leading to medial smooth muscle hyperplasia. Previous studies have suggested that CD26/dipeptidyl peptidase-4 (DPP4) plays a crucial role in the pathobiological processes in lung diseases. However, its role in pulmonary fibroblasts in hypoxic PH remains unknown. Therefore, we aimed to clarify the mechanistic role of CD26/DPP4 in lung fibroblasts in hypoxic PH. Dpp4 knockout (Dpp4 KO) and wild-type (WT) mice were exposed to hypoxia for 4 weeks. The degree of PH severity and medial wall thickness was augmented in Dpp4 KO mice compared with that in WT mice, suggesting that CD26/DPP4 plays a suppressive role in the development of hypoxic PH. Transcriptome analysis of human lung fibroblasts cultured under hypoxic conditions revealed that TGFB2, TGFB3, and TGFA were all upregulated as differentially expressed genes after DPP4 knockdown with small interfering RNA treatment. These results suggest that CD26/DPP4 plays a suppressive role in TGFβ signal-regulated fibroblast activation under hypoxic conditions. Therefore, CD26/DPP4 may be a potential therapeutic target in patients with PH associated with chronic hypoxia.
Keywords: CD26; DPP4; TGFβ; dipeptidyl peptidase-4; fibroblast; hypoxia; vascular remodeling.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Functional roles of CD26/DPP4 in bleomycin-induced pulmonary fibrosis.Physiol Rep. 2023 Mar;11(6):e15645. doi: 10.14814/phy2.15645. Physiol Rep. 2023. PMID: 36949656 Free PMC article.
-
Functional Roles of CD26/DPP4 in Bleomycin-Induced Pulmonary Hypertension Associated with Interstitial Lung Disease.Int J Mol Sci. 2024 Jan 6;25(2):748. doi: 10.3390/ijms25020748. Int J Mol Sci. 2024. PMID: 38255821 Free PMC article.
-
SOX9 promotes hypoxic pulmonary hypertension through stabilization of DPP4 in pulmonary artery smooth muscle cells.Exp Cell Res. 2024 Oct 1;442(2):114254. doi: 10.1016/j.yexcr.2024.114254. Epub 2024 Sep 12. Exp Cell Res. 2024. PMID: 39276964
-
Targeting cluster of differentiation 26 / dipeptidyl peptidase 4 (CD26/DPP4) in organ fibrosis.Br J Pharmacol. 2023 Nov;180(22):2846-2861. doi: 10.1111/bph.15967. Epub 2022 Nov 1. Br J Pharmacol. 2023. PMID: 36196001 Review.
-
Cut to the chase: a review of CD26/dipeptidyl peptidase-4's (DPP4) entanglement in the immune system.Clin Exp Immunol. 2016 Jul;185(1):1-21. doi: 10.1111/cei.12781. Epub 2016 May 13. Clin Exp Immunol. 2016. PMID: 26919392 Free PMC article. Review.
Cited by
-
Vascular Remodeling: The Multicellular Mechanisms of Pulmonary Hypertension.Int J Mol Sci. 2025 Apr 30;26(9):4265. doi: 10.3390/ijms26094265. Int J Mol Sci. 2025. PMID: 40362501 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
- A-5/GSK Japan Research Grant 2019
- 2019-G6/Therapeutics Research Initiative Grant from Chiba University School of Medicine
- 223fa627003h/AMED
- JP21gm1210003, JP23gm1810009/AMED-CREST
- 20FC1027, 23FC1031/Initiative for Realizing Diversity in the Research Environment; and a research grant from the In-tractable Respiratory Diseases and Pulmonary Hypertension Research Group, Ministry of Health, Labor and Welfare, Japan
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

