Blue Light-Induced Mitochondrial Oxidative Damage Underlay Retinal Pigment Epithelial Cell Apoptosis
- PMID: 39684332
- PMCID: PMC11641757
- DOI: 10.3390/ijms252312619
Blue Light-Induced Mitochondrial Oxidative Damage Underlay Retinal Pigment Epithelial Cell Apoptosis
Abstract
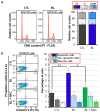
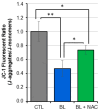
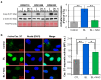
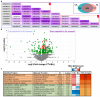
Reactive oxygen species (ROS) play a pivotal role in apoptosis. We reported that Blue Light (BL) induced oxidative stress in human retinal pigment epithelial (RPE) cells in vitro and increased drusen deposition and RPE cell apoptosis in human eyes. Here, we investigated the mechanisms underlying BL-induced damage to RPE cells. Cells were exposed to BL with or without the antioxidant N-acetylcysteine. Cells were analyzed for levels of ROS, proliferation, viability, and mitochondria membrane potential (ΔΨM) fluctuation. We performed proteomic analyses to search for differentially expressed proteins. ROS levels increased following RPE cell exposure to BL. While ROS production did not affect RPE cell proliferation, it was accompanied by decreased ΔΨM and increased cell apoptosis due to the caspase cascade activation in a ROS-dependent manner. Proteomic analyses revealed that BL decreased the levels of ROS detoxifying enzymes in exposed cells. We conclude that BL-induced oxidative stress is cytotoxic to RPE cells. These findings bring new insights into the involvement of BL on RPE cell damage and its role in the progression of age-related macular degeneration. The use of antioxidants is an avenue to block or delay BL-mediated RPE cell apoptosis to counteract the disease progression.
Keywords: antioxidant; apoptosis; blue light; caspases activation; mitochondria damage; oxidative stress; retinal pigment epithelial cells.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Wong W.L., Su X., Li X., Cheung C.M., Klein R., Cheng C.Y., Wong T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Health. 2014;2:e106–e116. doi: 10.1016/S2214-109X(13)70145-1. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

