Backstage Heroes-Yeast in COVID-19 Research
- PMID: 39684373
- PMCID: PMC11640846
- DOI: 10.3390/ijms252312661
Backstage Heroes-Yeast in COVID-19 Research
Abstract
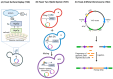
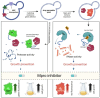
The extremely rapid development of understanding and technology that led to the containment of the COVID-19 pandemic resulted from collaborative efforts in the fields of Betacoronavirus pandemicum (SARS-CoV-2) biology, pharmacology, vaccinology, and medicine. Perhaps surprisingly, much of the research was conducted using simple and efficient yeast models. In this manuscript, we describe how yeast, eukaryotic microorganisms, have been used to research this global challenge, focusing on the therapeutic potential of the studies discussed herein. Thus, we outline the role of yeast in studying viral protein interactions with the host cell proteome, including the binding of the SARS-CoV-2 virus spike protein to the human ACE2 receptor and its modulation. The production and exploration of viral antigens in yeast systems, which led to the development of two approved COVID-19 vaccines, are also detailed. Moreover, yeast platforms facilitating the discovery and production of single-domain antibodies (nanobodies) against SARS-CoV-2 are described. Methods guiding modern and efficient drug discovery are explained at length. In particular, we focus on studies designed to search for inhibitors of the main protease (Mpro), a unique target for anti-coronaviral therapies. We highlight the adaptability of the techniques used, providing opportunities for rapid modification and implementation alongside the evolution of the SARS-CoV-2 virus. Approaches introduced in yeast systems that may have universal potential application in studies of emerging viral diseases are also described.
Keywords: COVID-19; SARS-CoV-2; Saccharomyces cerevisiae; vaccines; yeast surface display; yeast two-hybrid system.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures


Similar articles
-
Escape from neutralizing antibodies by SARS-CoV-2 spike protein variants.Elife. 2020 Oct 28;9:e61312. doi: 10.7554/eLife.61312. Elife. 2020. PMID: 33112236 Free PMC article.
-
Severe acute respiratory syndrome-coronavirus-2: Current advances in therapeutic targets and drug development.Rev Med Virol. 2021 May;31(3):e2174. doi: 10.1002/rmv.2174. Epub 2020 Sep 23. Rev Med Virol. 2021. PMID: 32965078 Free PMC article. Review.
-
Gold Metallodrugs to Target Coronavirus Proteins: Inhibitory Effects on the Spike-ACE2 Interaction and on PLpro Protease Activity by Auranofin and Gold Organometallics*.Chemistry. 2020 Nov 26;26(66):15140-15144. doi: 10.1002/chem.202004112. Epub 2020 Oct 19. Chemistry. 2020. PMID: 32915473 Free PMC article.
-
Potential therapeutic approaches for the early entry of SARS-CoV-2 by interrupting the interaction between the spike protein on SARS-CoV-2 and angiotensin-converting enzyme 2 (ACE2).Biochem Pharmacol. 2021 Oct;192:114724. doi: 10.1016/j.bcp.2021.114724. Epub 2021 Aug 8. Biochem Pharmacol. 2021. PMID: 34371003 Free PMC article. Review.
-
An automated positive selection screen in yeast provides support for boron-containing compounds as inhibitors of SARS-CoV-2 main protease.Microbiol Spectr. 2024 Oct 3;12(10):e0124924. doi: 10.1128/spectrum.01249-24. Epub 2024 Aug 20. Microbiol Spectr. 2024. PMID: 39162260 Free PMC article.
References
-
- Kashani N.R., Azadbakht J., Ehteram H., Kashani H.H., Rajabi-Moghadam H., Ahmad E., Nikzad H., Hosseini E.S. Molecular and Clinical Investigation of COVID-19: From Pathogenesis and Immune Responses to Novel Diagnosis and Treatment. Front. Mol. Biosci. 2022;9:770775. doi: 10.3389/fmolb.2022.770775. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

