Genetic Manipulation of Caveolin-1 in a Transgenic Mouse Model of Aortic Root Aneurysm: Sex-Dependent Effects on Endothelial and Smooth Muscle Function
- PMID: 39684412
- PMCID: PMC11641669
- DOI: 10.3390/ijms252312702
Genetic Manipulation of Caveolin-1 in a Transgenic Mouse Model of Aortic Root Aneurysm: Sex-Dependent Effects on Endothelial and Smooth Muscle Function
Abstract
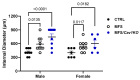
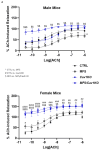
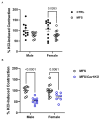
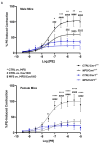
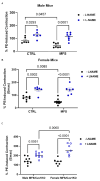
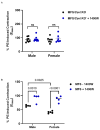
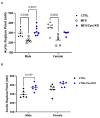
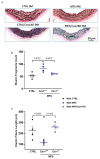
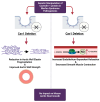
Marfan syndrome (MFS) is a systemic connective tissue disorder stemming from mutations in the gene encoding Fibrillin-1 (Fbn1), a key extracellular matrix glycoprotein. This condition manifests with various clinical features, the most critical of which is the formation of aortic root aneurysms. Reduced nitric oxide (NO) production due to diminished endothelial nitric oxide synthase (eNOS) activity has been linked to MFS aortic aneurysm pathology. Caveolin-1 (Cav1), a structural protein of plasma membrane caveolae, is known to inhibit eNOS activity, suggesting its involvement in MFS aneurysm progression by modulating NO levels. In this study, we examined the role of Cav1 in aortic smooth muscle and endothelial function, aortic wall elasticity, and wall strength in male and female MFS mice (FBN1+/Cys1041Gly) by generating developing Cav1-deficient MFS mice (MFS/Cav1KO). Our findings reveal that Cav1 ablation leads to a pronounced reduction in aortic smooth muscle contraction in response to phenylephrine, attributable to an increase in NO production in the aortic wall. Furthermore, we observed enhanced aortic relaxation responses to acetylcholine in MFS/Cav1KO mice, further underscoring Cav1's inhibitory impact on NO synthesis within the aorta. Notably, van Gieson staining and chamber myography analyses showed improved elastin fiber structure and wall strength in male MFS/Cav1KO mice, whereas these effects were absent in female counterparts. Cav1's regulatory influence on aortic root aneurysm development in MFS through NO-mediated modulation of smooth muscle and endothelial function, with notable sex-dependent variations.
Keywords: Marfan syndrome; aortic aneurysm; caveolin-1; endothelium; smooth muscle.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

