Extracellular Vesicles from a Novel Chordoma Cell Line, ARF-8, Promote Tumorigenic Microenvironmental Changes When Incubated with the Parental Cells and with Human Osteoblasts
- PMID: 39684443
- PMCID: PMC11641215
- DOI: 10.3390/ijms252312731
Extracellular Vesicles from a Novel Chordoma Cell Line, ARF-8, Promote Tumorigenic Microenvironmental Changes When Incubated with the Parental Cells and with Human Osteoblasts
Abstract
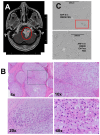
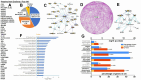
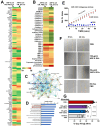
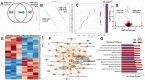
Chordomas are rare, generally slow-growing spinal tumors that nonetheless exhibit progressive characteristics over time, leading to malignant phenotypes and high recurrence rates, despite maximal therapeutic interventions. The tumors are notoriously resistant to therapies and are often located in regions that complicate achieving gross total resections. Cell lines from these tumors are rare as well. We cultured a new chordoma cell line (ARF-8) derived from an extensive clival chordoma that extended back to the cervical spine. We characterized the ARF-8 cellular and extracellular vesicle (EV) proteomes, as well as the impacts of ARF-8 EVs on the proteomes and secretomes of recipient cells (both ARF-8 and human osteoblasts) in autocrine and paracrine settings. Our proteomic analyses suggested roles for transforming growth factor beta (TGFB/TGFβ), cell-matrix interactions involving the epithelial-to-mesenchymal transition (EMT), and cell-extracellular matrix interactions in cell migration, consistent with a migratory/metastatic tumor phenotype. We demonstrated that ARF-8 tumor cell migration was dependent on general (arginine-glycine-aspartic acid [RGD]-based) integrin activity and that ARF-8 EVs could promote such migration. ARF-8 EVs also prompted proteomic/secretomic changes in human osteoblast cells, again with indications that cell-cell and cell-extracellular matrix interactions would be activated. All the characteristics typically associated with chordomas as cancers-migration and invasion, therapeutic resistance, metastatic potential-can be driven by tumor EVs. Overall, ARF-8 EVs promoted predicted tumorigenic phenotypes in recipient cells and suggested novel therapeutic targets for chordomas.
Keywords: cell line; chordoma; collagen; epithelial-to-mesenchymal transition (EMT); extracellular matrix; extracellular vesicles (EVs); integrins; migration; proteomics; secretome.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures



References
-
- International Agency for Research on Cancer . World Health Organization Classification of Tumours. 5th ed. Volume 3 IARC Publications; Lyon, France: 2020.
-
- International Agency for Research on Cancer . World Health Organization Classification of Tumours. 5th ed. Volume 3 IARC Publications; Lyon, France: 2020. WHO Classification of Tumours of Soft Tissue and Bone.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

