Nidogen 2 Overexpression Promotes Hepatosteatosis and Atherosclerosis
- PMID: 39684493
- PMCID: PMC11641205
- DOI: 10.3390/ijms252312782
Nidogen 2 Overexpression Promotes Hepatosteatosis and Atherosclerosis
Abstract
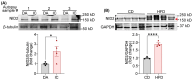
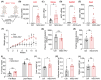
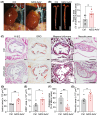
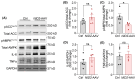
Clinical and genetic studies strongly support a significant connection between nonalcoholic fatty liver disease (NAFLD) and atherosclerotic cardiovascular disease (ASCVD) and identify ASCVD as the primary cause of death in NAFLD patients. Understanding the molecular factors and mechanisms regulating these diseases is critical for developing novel therapies that target them simultaneously. Our preliminary immunoblotting experiments demonstrated elevated expression of nidogen 2 (NID2), a basement membrane glycoprotein, in human atherosclerotic vascular tissues and murine steatotic livers. Therefore, we investigated the role of NID2 in regulating hepatosteatosis and atherosclerosis utilizing Western diet-fed Apoe-/- mice with/without NID2 overexpression. Quantitative real-time PCR confirmed increased NID2 mRNA expression in multiple organs (liver, heart, kidney, and adipose) of NID2-overexpressing mice. Male mice with NID2 overexpression exhibited higher liver and epididymal white adipose tissue mass, increased hepatic lipid accumulation, and fibrosis. Additionally, these mice developed larger atherosclerotic lesions in the whole aortas and aortic roots, with increased necrotic core formation. Mechanistic studies showed reduced AMPK activation in the livers of NID2-overexpressing mice compared with controls, without any effects on hepatic inflammation. In conclusion, these findings suggest that NID2 plays a deleterious role in both hepatosteatosis and atherosclerosis, making it a potential therapeutic target for these conditions.
Keywords: AMPK; NAFLD; atherosclerosis; hepatosteatosis; nidogen 2.
Conflict of interest statement
The authors have declared that no conflicts of interest exist.
Figures

References
-
- Riazi K., Azhari H., Charette J.H., Underwood F.E., King J.A., Afshar E.E., Swain M.G., Congly S.E., Kaplan G.G., Shaheen A.A. The prevalence and incidence of NAFLD worldwide: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2022;7:851–861. doi: 10.1016/S2468-1253(22)00165-0. - DOI - PubMed
-
- Loomba R., Ratziu V., Harrison S.A., NASH Clinical Trial Design International Working Group Expert Panel Review to Compare FDA and EMA Guidance on Drug Development and Endpoints in Nonalcoholic Steatohepatitis. Gastroenterology. 2022;162:680–688. doi: 10.1053/j.gastro.2021.10.051. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

