Mitochondrial Transplantation Ameliorates Pulmonary Fibrosis by Suppressing Myofibroblast Activation
- PMID: 39684495
- PMCID: PMC11641484
- DOI: 10.3390/ijms252312783
Mitochondrial Transplantation Ameliorates Pulmonary Fibrosis by Suppressing Myofibroblast Activation
Abstract
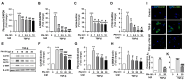
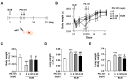
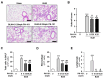
Idiopathic pulmonary fibrosis (IPF) is a pulmonary disease characterized by excessive extracellular matrix protein deposition in the lung interstitium, subsequently causing respiratory failure. IPF still has a high medical unmet requirement due to the lack of effective treatments to inhibit disease progression. The etiology of IPF remains unclear, but mitochondrial dysfunction is considered to be associated with IPF development. Therefore, targeting mitochondrial abnormalities would be a promising strategy for treating IPF. Recently, exogenous mitochondrial transplantation has been beneficial for treating mitochondrial dysfunction. The current study aimed to examine the therapeutic effect of mitochondrial transplantation on IPF in vitro and in vivo. Mitochondria were isolated from human umbilical cord mesenchymal stem cells, referred to as PN-101. Human lung fibroblasts and human bronchial epithelial cells were exposed to transforming growth factor-β, followed by PN-101 treatment to determine the in vitro efficacy of mitochondrial transplantation. An IPF mouse model established by a single intratracheal instillation of bleomycin was utilized to determine the in vivo efficacy of the intravenously treated mitochondria. PN-101 attenuated mitochondrial damage, inhibited EMC production, and suppressed epithelial-to-mesenchymal transition in vitro. Additionally, intravenous PN-101 administration alleviated bleomycin-induced fibrotic processes in the IPF mouse model with a therapeutic context. Our data indicate that PN-101 is a novel and potential therapeutic agent for IPF.
Keywords: anti-inflammation; antiapoptosis; idiopathic pulmonary fibrosis (IPF); mitochondria; stem cell; transplantation.
Conflict of interest statement
Seo-Eun Lee, Shin-Hye Yu, Young Cheol Kang, Yujin Kim, Jeong Seon Yeo, Jun Hyeok Lim, Iksun Kwon, Kuboem Han, and Chun-Hyung Kim were employed by the company PAEAN Biotechnology. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

