Combining antimiR-25 and cGAMP Nanocomplexes Enhances Immune Responses via M2 Macrophage Reprogramming
- PMID: 39684497
- PMCID: PMC11641323
- DOI: 10.3390/ijms252312787
Combining antimiR-25 and cGAMP Nanocomplexes Enhances Immune Responses via M2 Macrophage Reprogramming
Abstract
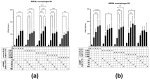
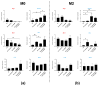
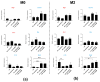
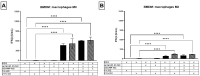
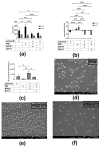
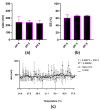
Glioblastoma (GBM) is an aggressive brain cancer with a highly immunosuppressive tumor microenvironment (TME), invariably infiltrated by tumor-associated macrophages (TAMs). These TAMs resemble M2 macrophages, which promote tumor growth and suppress immune responses. GBM cells secrete extracellular vesicles (EVs) containing microRNA-25, which inhibits the cGAS-STING pathway and prevents TAMs from adopting a pro-inflammatory M1 phenotype. This study characterizes antimiR-25/cGAMP nanocomplexes (NCs) for potential therapeutic applications. A particle size analysis revealed a significant reduction upon complexation with antimiR-25, resulting in smaller, more stable nanoparticles. Stability tests across pH levels (4-6) and temperatures (25-37 °C) demonstrated their resilience in various biological environments. Biological assays showed that antimiR-25 NCs interacted strongly with transferrin (Tf), suggesting potential for blood-brain barrier passage. The use of cGAMP NCs activated the cGAS-STING pathway in macrophages, leading to increased type I IFN (IFN-β) production and promoting a shift from the M2 to M1 phenotype. The combined use of cGAMP and antimiR-25 NCs also increased the expression of markers involved in M1 polarization. These findings offer insights into optimizing antimiR-25/cGAMP NCs for enhancing immune responses in GBM.
Keywords: EVs; PAMAM; STING pathway; antagomir-25; antimiR-25; cGAMP; cancer immunotherapy; extracellular vesicles; nanomedicine; polymeric nanoparticles.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Binnewies M., Roberts E.W., Kersten K., Chan V., Fearon D.F., Merad M., Coussens L.M., Gabrilovich D.I., Ostrand-Rosenberg S., Hedrick C.C., et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat. Med. 2018;24:541–550. doi: 10.1038/s41591-018-0014-x. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

