Coumarin Derivative Hybrids: Novel Dual Inhibitors Targeting Acetylcholinesterase and Monoamine Oxidases for Alzheimer's Therapy
- PMID: 39684512
- PMCID: PMC11641184
- DOI: 10.3390/ijms252312803
Coumarin Derivative Hybrids: Novel Dual Inhibitors Targeting Acetylcholinesterase and Monoamine Oxidases for Alzheimer's Therapy
Abstract
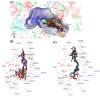
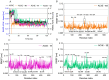
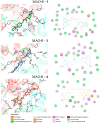
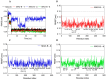
Multi-target-directed ligands (MTDLs) represent a promising frontier in tackling the complexity of multifactorial pathologies like Alzheimer's disease (AD). The synergistic inhibition of MAO-B, MAO-A, and AChE is believed to enhance treatment efficacy. A novel coumarin-based molecule substituted with O-phenylpiperazine via three- and four-carbon linkers at the 5- and 7-positions, has been identified as an effective MTDL against AD. Employing a medicinal chemistry approach, combined with molecular docking, molecular dynamic simulation, and ΔGbind estimation, two series of derivatives emerged as potent MTDLs: 8-acetyl-7-hydroxy-4-methylcoumarin (IC50: 1.52-4.95 μM for hAChE, 6.97-7.65 μM for hMAO-A) and 4,7-dimethyl-5-hydroxycoumarin (IC50: 1.88-4.76 μM for hMAO-B). They displayed binding free energy (ΔGbind) of -76.32 kcal/mol (11) and -70.12 kcal/mol (12) against AChE and -66.27 kcal/mol (11) and -62.89 kcal/mol (12) against MAO-A. It is noteworthy that compounds 11 and 12 demonstrated efficient binding to both AChE and MAO-A, while compounds 3 and 10 significantly reduced MAO-B and AChE aggregation in vitro. These findings provide structural templates for the development of dual MAO and AChE inhibitors for the treatment of neurodegenerative diseases.
Keywords: Alzheimer’s disease; coumarin derivatives; hAChE/hMAO-B dual-targeted inhibitor; molecular docking; molecular dynamic simulation.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






References
-
- Li Y., Qiang X., Luo L., Yang X., Xiao G., Liu Q., Ai J., Tan Z., Deng Y. Aurone Mannich base derivatives as promising multifunctional agents with acetylcholinesterase inhibition, anti-beta-amyloid aggragation and neuroprotective properties for the treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2017;126:762–775. doi: 10.1016/j.ejmech.2016.12.009. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

