Endothelial Dysfunction and Liver Cirrhosis: Unraveling of a Complex Relationship
- PMID: 39684569
- PMCID: PMC11640898
- DOI: 10.3390/ijms252312859
Endothelial Dysfunction and Liver Cirrhosis: Unraveling of a Complex Relationship
Abstract
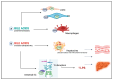
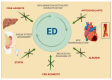
Endothelial dysfunction (ED) is the in the background of multiple metabolic diseases and a key process in liver disease progression and cirrhosis decompensation. ED affects liver sinusoidal endothelial cells (LSECs) in response to different damaging agents, causing their progressive dedifferentiation, unavoidably associated with an increase in intrahepatic resistance that leads to portal hypertension and hyperdynamic circulation with increased cardiac output and low peripheral artery resistance. These changes are driven by a continuous interplay between different hepatic cell types, invariably leading to increased reactive oxygen species (ROS) formation, increased release of pro-inflammatory cytokines and chemokines, and reduced nitric oxide (NO) bioavailability, with a subsequent loss of proper vascular tone regulation and fibrosis development. ED evaluation is often accomplished by serum markers and the flow-mediated dilation (FMD) measurement of the brachial artery to assess its NO-dependent response to shear stress, which usually decreases in ED. In the context of liver cirrhosis, the ED assessment could help understand the complex hemodynamic changes occurring in the early and late stages of the disease. However, the instauration of a hyperdynamic state and the different NO bioavailability in intrahepatic and systemic circulation-often defined as the NO paradox-must be considered confounding factors during FMD analysis. The primary purpose of this review is to describe the main features of ED and highlight the key findings of the dynamic and intriguing relationship between ED and liver disease. We will also focus on the significance of FMD evaluation in this setting, pointing out its key role as a therapeutic target in the never-ending battle against liver cirrhosis progression.
Keywords: Krüppel-like factor; albumin; cirrhosis; endothelial dysfunction; endothelium; flow-mediated dilation; nitric oxide; shear stress; statins.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

