Structure of Genes Encoding Oxidosqualene Cyclases-Key Enzymes of Triterpenoid Biosynthesis from Sea Cucumber Eupentacta fraudatrix
- PMID: 39684591
- PMCID: PMC11641436
- DOI: 10.3390/ijms252312881
Structure of Genes Encoding Oxidosqualene Cyclases-Key Enzymes of Triterpenoid Biosynthesis from Sea Cucumber Eupentacta fraudatrix
Abstract
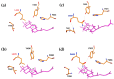
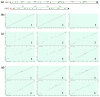
Oxidosqualene cyclases (OSCs) are enzymes responsible for converting linear triterpenes into tetracyclic ones, which are known as precursors of other important and bioactive metabolites. Two OSCs genes encoding parkeol synthase and lanostadienol synthase have been found in representatives of the genera Apostichopus and Stichopus (family Stichopodidae, order Synallactida). As a limited number of sea cucumber OSCs have been studied thus far, OSCs encoding gene(s) of the sea cucumber Eupentacta fraudatrix (family Sclerodactylidae, order Dendrochirotida) were investigated to fill this gap. Here, we employed RACEs, molecular cloning, and Oxford Nanopore Technologies to identify candidate OSC mRNAs and genes. The assembled cDNAs were 2409 bp (OSC1) and 3263 bp (OSC2), which shared the same CDS size of 2163 bp encoding a 721-amino-acid protein. The E. fraudatrix OSC1 and OSC2 had higher sequence identity similarity to each other (77.5%) than to other holothurian OSCs (64.7-71.0%). According to the sequence and molecular docking analyses, OSC1 with L436 is predicted to be parkeol synthase, while OSC2 with Q439 is predicted to be lanostadienol synthase. Based on the phylogenetic analysis, E. fraudatrix OSCs cDNAs clustered with other holothurian OSCs, forming the isolated branch. As a result of gene analysis, the high polymorphism and larger size of the OSC1 gene suggest that this gene may be an ancestor of the OSC2 gene. These results imply that the E. fraudatrix genome contains two OSC genes whose evolutionary pathways are different from those of the OSC genes in Stichopodidae.
Keywords: 2,3-oxydosqualene cyclase; Eupentacta fraudatrix; gene determination and analysis; molecular docking; phylogeny.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Dyakonov A., Baranova Z., Saveleva T. Note on Holothurioidea of the South Sakhalin and South Kurile Islands area. Investig. Seas USSR. 1958;5:358–380.
-
- Silchenko A.S., Kalinovsky A.I., Avilov S.A., Andryjaschenko P.V., Dmitrenok P.S., Menchinskaya E.S., Kalinin V.I. Structure of cucumarioside I2 from the sea cucumber Eupentacta fraudatrix (Djakonov et Baranova) and cytotoxic and immunostimulatory activities of this saponin and relative compounds. Nat. Prod. Res. 2013;27:1776–1783. doi: 10.1080/14786419.2013.778851. - DOI - PubMed
-
- Silchenko A.S., Kalinovsky A.I., Avilov S.A., Andryjaschenko P.V., Dmitrenok P.S., Kalinin V.I., Stonik V.A. 3β-O-Glycosylated 16β-acetoxy-9β-H-lanosta-7,24-diene-3β,18,20β-triol, an intermediate metabolite from the sea cucumber Eupentacta fraudatrix and its biosynthetic significance. Biochem. Syst. Ecol. 2012;44:53–60. doi: 10.1016/j.bse.2012.04.008. - DOI
-
- Silchenko A.S., Kalinovsky A.I., Avilov S.A., Andryjaschenko P.V., Dmitrenok P.S., Martyyas E.A., Kalinin V.I. Triterpene glycosides from the sea cucumber Eupentacta fraudatrix. Structure and biological action of cucumariosides A1, A3, A4, A5, A6, A12 and A15, seven new minor non-sulfated tetraosides and unprecedented 25-keto, 27-norholostane aglycone. Nat. Prod. Commun. 2012;7:517–525. doi: 10.1177/1934578X1200700426. - DOI - PubMed
-
- Silchenko A.S., Kalinovsky A.I., Avilov S.A., Andryjaschenko P.V., Dmitrenok P.S., Martyyas E.A., Kalinin V.I. Triterpene Glycosides from the Sea Cucumber Eupentacta fraudatrix. Structure and Biological Action of Cucumariosides I1, I3, I4, Three New Minor Disulfated Pentaosides. Nat. Prod. Commun. 2013;8:1053–1058. doi: 10.1177/1934578X1300800805. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

