A Reduction in Mitophagy Is Associated with Glaucomatous Neurodegeneration in Rodent Models of Glaucoma
- PMID: 39684751
- PMCID: PMC11642561
- DOI: 10.3390/ijms252313040
A Reduction in Mitophagy Is Associated with Glaucomatous Neurodegeneration in Rodent Models of Glaucoma
Abstract
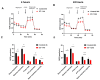
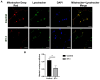
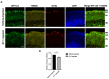
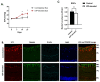
Glaucoma is a heterogenous group of optic neuropathies characterized by the degeneration of optic nerve axons and the progressive loss of retinal ganglion cells (RGCs), which could ultimately lead to vision loss. Elevated intraocular pressure (IOP) is a major risk factor in the development of glaucoma, and reducing IOP remains the main therapeutic strategy. Endothelin-1 (ET-1), a potent vasoactive peptide, has been shown to produce neurodegenerative effects in animal models of glaucoma. However, the detailed mechanisms underlying ET-1-mediated neurodegeneration in glaucoma are not completely understood. In the current study, using a Seahorse Mitostress assay, we report that ET-1 treatment for 4 h and 24 h time points causes a significant decline in various parameters of mitochondrial function, including ATP production, maximal respiration, and spare respiratory capacity in cultured RGCs. This compromise in mitochondrial function could trigger activation of mitophagy as a quality control mechanism to restore RGC health. Contrary to our expectation, we observed a decrease in mitophagy following ET-1 treatment for 24 h in cultured RGCs. Using Morrison's model of ocular hypertension in rats, we investigated here, for the first time, changes in mitophagosome formation by analyzing the co-localization of LC-3B and TOM20 in RGCs. We also injected ET-1 (24 h) into transgenic GFP-LC3 mice to analyze the formation of mitophagosomes in vivo. In Morrison's model of ocular hypertension, as well as in ET-1 injected GFP-LC3 mice, we found a decrease in co-localization of LC3 and TOM20, indicating reduced mitophagy. Taken together, these results demonstrate that both ocular hypertension and ET-1 administration in rats and mice lead to reduced mitophagy, thus predisposing RGCs to neurodegeneration.
Keywords: glaucoma; mitophagy; neurodegeneration.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

