Inflammasomes in Intestinal Disease: Mechanisms of Activation and Therapeutic Strategies
- PMID: 39684769
- PMCID: PMC11642578
- DOI: 10.3390/ijms252313058
Inflammasomes in Intestinal Disease: Mechanisms of Activation and Therapeutic Strategies
Abstract
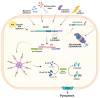
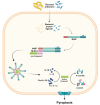
NOD-like receptors (NLRs) are a family of cytosolic pattern recognition receptors (PRRs) implicated in the innate immune sensing of pathogens and damage signals. NLRs act as sensors in multi-protein complexes called inflammasomes. Inflammasome activity is necessary for the maintenance of intestinal homeostasis, although their aberrant activation contributes to the pathogenesis of several gastrointestinal diseases. In this review, we summarize the main features of the predominant types of inflammasomes involved in gastrointestinal immune responses and their implications in intestinal disease, including Irritable Bowel Syndrome (IBS), Inflammatory Bowel Disease (IBD), celiac disease, and Colorectal Cancer (CRC). In addition, we report therapeutic discoveries that target the inflammasome pathway, highlighting promising novel therapeutic strategies in the treatment of intestinal diseases. Collectively, our understanding of the mechanisms of intestinal inflammasome activation and their interactions with other immune pathways appear to be not fully elucidated. Moreover, the clinical relevance of the efficacy of inflammasome inhibitors has not been evaluated. Despite these limitations, a greater understanding of the effectiveness, specificity, and reliability of pharmacological and natural inhibitors that target inflammasome components could be an opportunity to develop new therapeutic options for the treatment of intestinal disease.
Keywords: colorectal cancer; gastrointestinal disease; inflammasome; inflammatory bowel disease; miRNAs.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

