Novel Mutation Lys30Glu in the TPM1 Gene Leads to Pediatric Left Ventricular Non-Compaction and Dilated Cardiomyopathy via Impairment of Structural and Functional Properties of Cardiac Tropomyosin
- PMID: 39684770
- PMCID: PMC11641563
- DOI: 10.3390/ijms252313059
Novel Mutation Lys30Glu in the TPM1 Gene Leads to Pediatric Left Ventricular Non-Compaction and Dilated Cardiomyopathy via Impairment of Structural and Functional Properties of Cardiac Tropomyosin
Abstract
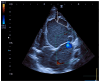
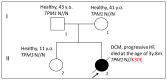
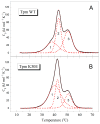
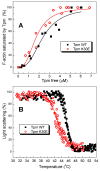
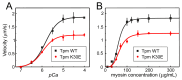
Pediatric dilated cardiomyopathy (DCM) is a rare heart muscle disorder leading to the enlargement of all chambers and systolic dysfunction. We identified a novel de novo variant, c.88A>G (p.Lys30Glu, K30E), in the TPM1 gene encoding the major cardiac muscle tropomyosin (Tpm) isoform, Tpm1.1. The variant was found in a proband with DCM and left ventricular non-compaction who progressed to terminal heart failure at the age of 3 years and 8 months. To study the properties of the mutant protein, we produced recombinant K30E Tpm and used various biochemical and biophysical methods to compare its properties with those of WT Tpm. The K30E substitution decreased the thermal stability of Tpm and its complex with actin and significantly reduced the sliding velocity of the regulated thin filaments over a surface covered by ovine cardiac myosin in an in vitro motility assay across the entire physiological range of Ca2+ concentration. Our molecular dynamics simulations suggest that the charge reversal of the 30th residue of Tpm alters the actin monomer to which it is bound. We hypothesize that this rearrangement of the actin-Tpm interaction may hinder the transition of a myosin head attached to a nearby actin from a weakly to a strongly bound, force-generating state, thereby reducing myocardial contractility. The impaired myosin interaction with regulated actin filaments and the decreased thermal stability of the actin-Tpm complex at a near physiological temperature likely contribute to the pathogenicity of the variant and its causative role in progressive DCM.
Keywords: actin–myosin interaction; dilated cardiomyopathy; in vitro motility assay; left ventricular non-compaction; molecular dynamics; tropomyosin.
Conflict of interest statement
Natalia S. Ryabkova and Ivan A. Katrukha were employed by HyTest Ltd. The authors declare that this research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

References
-
- Mestroni L., Maisch B., McKenna W.J., Schwartz K., Charron P., Rocco C., Tesson F., Richter A., Wilke A., Komajda M. Guidelines for the study of familial dilated cardiomyopathies. Collaborative Research Group of the European Human and Capital Mobility Project on Familial Dilated Cardiomyopathy. Eur. Heart J. 1999;20:93–102. doi: 10.1053/euhj.1998.1145. - DOI - PubMed
-
- Hershberger R.E., Givertz M.M., Ho C.Y., Judge D.P., Kantor P.F., McBride K.L., Morales A., Taylor M.R., Vatta M., Ware S.M. Genetic evaluation of cardiomyopathy-A Heart Failure Society of America Practice Guideline. J. Card. Fail. 2018;24:281–302. doi: 10.1016/j.cardfail.2018.03.004. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

