Single-Cell RNA Sequencing of PBMCs Identified Junction Plakoglobin (JUP) as Stratification Biomarker for Endometriosis
- PMID: 39684780
- PMCID: PMC11641628
- DOI: 10.3390/ijms252313071
Single-Cell RNA Sequencing of PBMCs Identified Junction Plakoglobin (JUP) as Stratification Biomarker for Endometriosis
Abstract
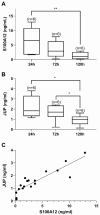
This study aimed to identify unique characteristics in the peripheral blood mononuclear cells (PBMCs) of endometriosis patients and develop a non-invasive early diagnostic tool. Using single-cell RNA sequencing (scRNA-seq), we constructed the first single-cell atlas of PBMCs from endometriosis patients based on 107,964 cells and 25,847 genes. Within CD16+ monocytes, we discovered JUP as a dysregulated gene. To assess its diagnostic potential, we measured peritoneal fluid (PF) and serum JUP levels in a large cohort of 199 patients including 20 women with ovarian cancer (OC). JUP was barely detectable in PF but was significantly elevated in the serum of patients with endometriosis and OC, with levels 1.33 and 2.34 times higher than controls, respectively. Additionally, JUP was found in conditioned culture media of CD14+/CD16+ monocytes aligning with our scRNA-seq data. Serum JUP levels correlated with endometriosis severity and endometrioma presence but were unaffected by dysmenorrhea, menstrual cycle, or adenomyosis. When combined with CA125 (cancer antigen 125) JUP enhanced the specificity of endometriosis diagnosis from 89.13% (CA125 measured alone) to 100%. While sensitivity remains a challenge at 19%, our results suggest that JUP's potential to enhance diagnostic accuracy warrants additional investigation. Furthermore, employing serum JUP as a stratification marker unlocked the potential to identify additional endometriosis-related genes, offering novel insights into disease pathogenesis.
Keywords: Junctional plakoglobin (JUP); adenomyosis; diagnostic biomarkers; endometriosis; peripheral blood mononuclear cells (PBMCs); single-cell RNA-sequencing (scRNA-seq).
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Wentzensen N., Poole E.M., Trabert B., White E., Arslan A.A., Patel A.V., Setiawan V.W., Visvanathan K., Weiderpass E., Adami H.-O., et al. Ovarian Cancer Risk Factors by Histologic Subtype: An Analysis From the Ovarian Cancer Cohort Consortium. J. Clin. Oncol. 2016;34:2888–2898. doi: 10.1200/JCO.2016.66.8178. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

