Neuroprotective and Anti-Inflammatory Effects of Dimethyl Fumarate, Monomethyl Fumarate, and Cannabidiol in Neurons and Microglia
- PMID: 39684792
- PMCID: PMC11642486
- DOI: 10.3390/ijms252313082
Neuroprotective and Anti-Inflammatory Effects of Dimethyl Fumarate, Monomethyl Fumarate, and Cannabidiol in Neurons and Microglia
Abstract
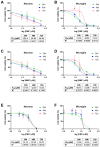
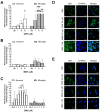
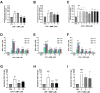
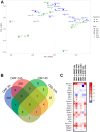
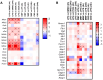
Dimethyl fumarate (DMF) is an immunomodulatory treatment for multiple sclerosis (MS) that can cross the blood-brain barrier, presenting neuroprotective potential. Its mechanism of action is not fully understood, and there is a need to characterize whether DMF or its bioactive metabolite monomethyl fumarate (MMF) exerts neuroprotective properties. Moreover, the combination of adjuvant agents such as cannabidiol (CBD) could be relevant to enhance neuroprotection. The aim of this study was to compare the neuroprotective and immunomodulatory effects of DMF, MMF, and CBD in neurons and microglia in vitro. We found that DMF and CBD, but not MMF, activated the Nrf2 antioxidant pathway in neurons. Similarly, only DMF and CBD, but not MMF, prevented the LPS-induced activation of the inflammatory pathway NF-kB in microglia. Additionally, the three drugs inhibited the production of nitric oxide in microglia and protected neurons against apoptosis. Transcriptomically, DMF modulated a greater number of inflammatory and Nrf2-related genes compared to MMF and CBD in both neurons and microglia. Our results show that DMF and MMF, despite being structurally related, present differences in their mechanisms of action that could be relevant for the achievement of neuroprotection in MS patients. Additionally, CBD shows potential as a neuroprotective agent.
Keywords: NF-kB; Nrf2; cannabidiol; dimethyl fumarate; microglia; monomethyl fumarate; multiple sclerosis; neuron; neuroprotection; transcriptome.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Mrowietz U., Barker J., Boehncke W.H., Iversen L., Kirby B., Naldi L., Reich K., Tanew A., van de Kerkhof P.C.M., Warren R.B. Clinical use of dimethyl fumarate in moderate-to-severe plaque-type psoriasis: A European expert consensus. J. Eur. Acad. Dermatol. Venereol. 2018;32:3–14. doi: 10.1111/jdv.15218. - DOI - PubMed
-
- European Medicines Agency Tecfidera 120 mg Gastro-Resistant Hard Capsules. [(accessed on 30 October 2024)]. Available online: https://www.ema.europa.eu/documents/product-information/tecfidera-epar-p....
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

