Variations in BDNF and Their Role in the Neurotrophic Antidepressant Mechanisms of Ketamine and Esketamine: A Review
- PMID: 39684808
- PMCID: PMC11642200
- DOI: 10.3390/ijms252313098
Variations in BDNF and Their Role in the Neurotrophic Antidepressant Mechanisms of Ketamine and Esketamine: A Review
Abstract
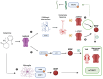
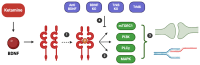
Brain-derived neurotrophic factor (BDNF) is critical for neuroplasticity, synaptic transmission, and neuronal survival. Studies have implicated it in the pathophysiology of depression, as its expression is significantly reduced in brain areas such as the prefrontal cortex and hippocampus in patients with depression. Our narrative review focuses on the relationship between BDNF, ketamine, and esketamine, specifically by summarizing human studies investigating BDNF variations in patients treated with these two drugs. BDNF plays a pivotal role in neuroplasticity and neurotrophic mechanisms that can be enhanced by traditional antidepressants, which have been shown to increase BDNF levels both peripherally and in targeted brain regions. Ketamine and its S-enantiomer, esketamine, exert both rapid and sustained antidepressant effects through activation of glutamate-related pathways, with neurotrophic effects involving BDNF, as demonstrated in experimental studies. However, clinical findings have shown mixed results, with most indicating an increase in plasma BDNF in patients treated with intravenous ketamine, although some studies contradict these findings. In addition to this, there are few studies of BDNF and esketamine. Currently, the limited number of studies suggests the need for further research, including larger sample sizes and investigations of BDNF and intranasal esketamine, which has been approved by several regulatory agencies for the treatment of treatment-resistant depression.
Keywords: BDNF; esketamine; ketamine; major depressive disorder; treatment-resistant depression.
Conflict of interest statement
Andrea Fagiolini has received research grants and/or has been a consultant for, and/or has been a speaker for: Allergan, Angelini, Apsend, Generici DOC, Lundbeck, Italfar-maco, Janssen, Otsuka, Pfizer, Recordati, Roche, Sanofi Aventis, Sunovion; Alessandro Cuomo is/has been a consultant and/or a speaker for Angelini, Glaxo Smith Kline, Lundbeck, Janssen, Otsuka, Pfizer, Recordati.
Figures

References
-
- Cavaleri D., Moretti F., Bartoccetti A., Mauro S., Crocamo C., Carrà G., Bartoli F. The Role of BDNF in Major Depressive Disorder, Related Clinical Features, and Antidepressant Treatment: Insight from Meta-Analyses. Neurosci. Biobehav. Rev. 2023;149:105159. doi: 10.1016/j.neubiorev.2023.105159. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

