Development of an rpS6-Based Ex Vivo Assay for the Analysis of Neuronal Activity in Mouse and Human Olfactory Systems
- PMID: 39684883
- PMCID: PMC11642283
- DOI: 10.3390/ijms252313173
Development of an rpS6-Based Ex Vivo Assay for the Analysis of Neuronal Activity in Mouse and Human Olfactory Systems
Abstract
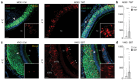
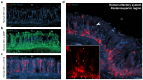
Olfactory sensitivity to odorant molecules is a complex biological function influenced by both endogenous factors, such as genetic background and physiological state, and exogenous factors, such as environmental conditions. In animals, this vital ability is mediated by olfactory sensory neurons (OSNs), which are distributed across several specialized olfactory subsystems depending on the species. Using the phosphorylation of the ribosomal protein S6 (rpS6) in OSNs following sensory stimulation, we developed an ex vivo assay allowing the simultaneous conditioning and odorant stimulation of different mouse olfactory subsystems, including the main olfactory epithelium, the vomeronasal organ, and the Grueneberg ganglion. This approach enabled us to observe odorant-induced neuronal activity within the different olfactory subsystems and to demonstrate the impact of environmental conditioning, such as temperature variations, on olfactory sensitivity, specifically in the Grueneberg ganglion. We further applied our rpS6-based assay to the human olfactory system and demonstrated its feasibility. Our findings show that analyzing rpS6 signal intensity is a robust and highly reproducible indicator of neuronal activity across various olfactory systems, while avoiding stress and some experimental limitations associated with in vivo exposure. The potential extension of this assay to other conditioning paradigms and olfactory systems, as well as its application to other animal species, including human olfactory diagnostics, is also discussed.
Keywords: 3Rs; Grueneberg ganglion; environmental factors; human; mouse; neuronal activity; olfaction; olfactory subsystems; rpS6.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
CD36 is expressed in a defined subpopulation of neurons in the olfactory epithelium.Sci Rep. 2016 May 5;6:25507. doi: 10.1038/srep25507. Sci Rep. 2016. PMID: 27145700 Free PMC article.
-
Sodium/calcium exchanger expression in the mouse and rat olfactory systems.J Comp Neurol. 2007 Apr 20;501(6):944-58. doi: 10.1002/cne.21290. J Comp Neurol. 2007. PMID: 17311327
-
Role of iRhom2 in Olfaction: Implications for Odorant Receptor Regulation and Activity-Dependent Adaptation.Int J Mol Sci. 2024 May 31;25(11):6079. doi: 10.3390/ijms25116079. Int J Mol Sci. 2024. PMID: 38892263 Free PMC article.
-
Of pheromones and kairomones: what receptors mediate innate emotional responses?Anat Rec (Hoboken). 2013 Sep;296(9):1346-63. doi: 10.1002/ar.22745. Epub 2013 Jul 31. Anat Rec (Hoboken). 2013. PMID: 23904448 Review.
-
Detection of odorants through the main olfactory epithelium and vomeronasal organ of mice.Nutr Rev. 2004 Nov;62(11 Pt 2):S189-92; discussion S224-41. doi: 10.1111/j.1753-4887.2004.tb00098.x. Nutr Rev. 2004. PMID: 15630934 Review.
Cited by
-
Hydrogen sulfide as a potent predator-derived kairomone mediating fear-related responses in mice.Commun Biol. 2025 Aug 1;8(1):1144. doi: 10.1038/s42003-025-08592-w. Commun Biol. 2025. PMID: 40750867 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

