Real-Life Use of Filgotinib in Rheumatoid Arthritis: A Retrospective Cohort Study
- PMID: 39685644
- PMCID: PMC11642713
- DOI: 10.3390/jcm13237185
Real-Life Use of Filgotinib in Rheumatoid Arthritis: A Retrospective Cohort Study
Abstract
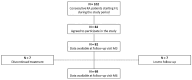
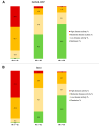
Background: Janus kinase inhibitors (JAKis) are a novel class of drugs interfering with intracellular signaling of type I and type II cytokines, which play a crucial role in immune dysregulation associated with several chronic inflammatory diseases. Filgotinib (FIL), in particular, is the newest member of the JAKi class and exerts its therapeutic effects by selectively targeting and inhibiting the kinase activity of JAK1. While the efficacy of FIL in rheumatoid arthritis (RA) has been confirmed in clinical trials, real-world evidence may provide better insights into its effectiveness and safety in routine clinical practice. Methods: We performed a multicenter, retrospective cohort study investigating the real-life effectiveness and safety of FIL in adult patients with RA. Demographic information, disease characteristics, prior treatment history, and comorbid conditions were retrieved from clinical records at baseline (M0) and after 3 (M3) and 6 months (M6) of treatment. Results: A total of 82 patients (63 women) agreed to participate in the study, of whom 39 (47.6%) were older than 65 years. The average RA duration was 13 ± 9 years; 19 patients (23.1%) were current or former smokers, and 4 patients (4.9%) had a history of cardiovascular events. Most patients had previously received at least one biologic disease-modifying antirheumatic drug (range: 1-6+); in addition, 11 patients (13.4%) had been already exposed to another JAKi. During the follow-up, 7 patients discontinued treatment due to primary failure (n = 3) or adverse events (n = 4). Significant reductions in pain and number of tender and swollen joints were observed at M3 and M6. A relevant proportion of patients achieved DAS28-CRP remission at M3 and M6 (46.3% and 66.2%, respectively). Conclusions: Our data provide additional insight into the effectiveness of filgotinib in a real-world setting, even among patients with difficult-to-treat RA and a high prevalence of cardiovascular risk factors.
Keywords: JAK inhibitors; filgotinib; rheumatoid arthritis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Smolen J.S., Landewé R.B.M., Bergstra S.A., Kerschbaumer A., Sepriano A., Aletaha D., Caporali R., Edwards C.J., Hyrich K.L., Pope J.E., et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2022 update. Ann. Rheum. Dis. 2023;82:3–18. doi: 10.1136/ard-2022-223356. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

