Clinical Validation of Respiratory Rate Estimation Using Acoustic Signals from a Wearable Device
- PMID: 39685655
- PMCID: PMC11642485
- DOI: 10.3390/jcm13237199
Clinical Validation of Respiratory Rate Estimation Using Acoustic Signals from a Wearable Device
Abstract
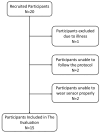
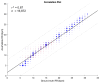
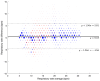
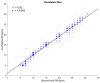
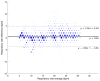
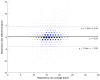
Objectives: Respiratory rate (RR) is a clinical measure of breathing frequency, a vital metric for clinical assessment. However, the recording and documentation of RR are considered to be extremely poor due to the limitations of the current approaches to measuring RR, including capnography and manual counting. We conducted a validation of the automatic RR measurement capability of AcuPebble RE100 (Acurable, London, UK) against a gold-standard capnography system and a type-III cardiorespiratory polygraphy system in two independent prospective and retrospective studies. Methods: The experiment for the prospective study was conducted at Imperial College London. Data from AcuPebble RE100 (Acurable, London, UK) and the reference capnography system (Capnostream™35, Medtronic, Minneapolis, MN, USA) were collected simultaneously from healthy volunteers. The data from a previously published study were used in the retrospective study, where the patients were recruited consecutively from a standard Obstructive Sleep Apnea (OSA) diagnostic pathway in a UK hospital. Overnight data during sleep were collected using the AcuPebble SA100 (Acurable, London, UK) sensor and a type-III cardiorespiratory polygraphy system (Embletta MPR Sleep System, Natus Medical, Pleasanton, CA, USA) at the patients' homes. Data from 15 healthy volunteers were used in the prospective study. For the retrospective study, 150 consecutive patients had been referred for OSA diagnosis and successfully completed the study. Results: The RR output of AcuPebble RE100 (Acurable, London, UK) was compared against the reference device in terms of the Root Mean Squared Deviation (RMSD), mean error, and standard deviation (SD) of the difference between the paired measurements. In both the prospective and retrospective studies, the AcuPebble RE100 algorithms provided accurate RR measurements, well within the clinically relevant margin of error, typically used by FDA-approved respiratory rate monitoring devices, with the RMSD under three breaths per minute (BPM) and mean errors of 1.83 BPM and 1.4 BPM, respectively. Conclusions: The evaluation results provide evidence that AcuPebble RE100 (Acurable, London, UK) algorithms produce reliable results and are hence suitable for overnight monitoring of RR.
Keywords: AcuPebble; digital health; respiratory rate; validation study; wearable technology.
Conflict of interest statement
ER-V is the Chief Scientific Officer and the founder of Acurable, the company that owns the AcuPebble RE100 systems. RXAP works part-time as a senior signal processing engineer at Acurable.
Figures

Similar articles
-
Accuracy and usability of AcuPebble SA100 for automated diagnosis of obstructive sleep apnoea in the home environment setting: an evaluation study.BMJ Open. 2021 Dec 21;11(12):e046803. doi: 10.1136/bmjopen-2020-046803. BMJ Open. 2021. PMID: 34933855 Free PMC article.
-
Validation of a Wearable Medical Device for Automatic Diagnosis of OSA against Standard PSG.J Clin Med. 2024 Jan 19;13(2):571. doi: 10.3390/jcm13020571. J Clin Med. 2024. PMID: 38276077 Free PMC article.
-
Economic impact case study of a wearable medical device for the diagnosis of obstructive sleep apnoea.BMC Health Serv Res. 2024 Nov 1;24(1):1337. doi: 10.1186/s12913-024-11694-6. BMC Health Serv Res. 2024. PMID: 39487472 Free PMC article.
-
Validation of Tracheal Sound-Based Respiratory Effort Monitoring for Obstructive Sleep Apnoea Diagnosis.J Clin Med. 2024 Jun 20;13(12):3628. doi: 10.3390/jcm13123628. J Clin Med. 2024. PMID: 38930155 Free PMC article.
-
Obstructive sleep apnea devices for out-of-center (OOC) testing: technology evaluation.J Clin Sleep Med. 2011 Oct 15;7(5):531-48. doi: 10.5664/JCSM.1328. J Clin Sleep Med. 2011. PMID: 22003351 Free PMC article. Review.
References
-
- National Institute for Health and Care Excellent (NICE) National Early Warning Score Systems That Alert to Deteriorating Adult Patients in Hospital. NICE Advice. [(accessed on 22 November 2024)]. Available online: https://www.nice.org.uk/advice/mib205.
Grants and funding
LinkOut - more resources
Full Text Sources

