Biochemical Sensors for Personalized Therapy in Parkinson's Disease: Where We Stand
- PMID: 39685917
- PMCID: PMC11641839
- DOI: 10.3390/jcm13237458
Biochemical Sensors for Personalized Therapy in Parkinson's Disease: Where We Stand
Abstract
Since its first introduction, levodopa has remained the cornerstone treatment for Parkinson's disease. However, as the disease advances, the therapeutic window for levodopa narrows, leading to motor complications like fluctuations and dyskinesias. Clinicians face challenges in optimizing daily therapeutic regimens, particularly in advanced stages, due to the lack of quantitative biomarkers for continuous motor monitoring. Biochemical sensing of levodopa offers a promising approach for real-time therapeutic feedback, potentially sustaining an optimal motor state throughout the day. These sensors vary in invasiveness, encompassing techniques like microdialysis, electrochemical non-enzymatic sensing, and enzymatic approaches. Electrochemical sensing, including wearable solutions that utilize reverse iontophoresis and microneedles, is notable for its potential in non-invasive or minimally invasive monitoring. Point-of-care devices and standard electrochemical cells demonstrate superior performance compared to wearable solutions; however, this comes at the cost of wearability. As a result, they are better suited for clinical use. The integration of nanomaterials such as carbon nanotubes, metal-organic frameworks, and graphene has significantly enhanced sensor sensitivity, selectivity, and detection performance. This framework paves the way for accurate, continuous monitoring of levodopa and its metabolites in biofluids such as sweat and interstitial fluid, aiding real-time motor performance assessment in Parkinson's disease. This review highlights recent advancements in biochemical sensing for levodopa and catecholamine monitoring, exploring emerging technologies and their potential role in developing closed-loop therapy for Parkinson's disease.
Keywords: Parkinson’s disease (PD); biochemical sensors; closed-loop therapy; levodopa.
Conflict of interest statement
Davide Ciarrocchi has no disclosures. Alessandro Zompanti has no disclosures. Giorgio Pennazza has no disclosures. Marco Santonico has no disclosures. Pasquale Maria Pecoraro has no disclosures. Lazzaro di Biase is the scientific director and one of the shareholders of Brain Innovations Srl, a University spinoff of Campus Bio-Medico University of Rome. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or conflict with the subject matter or materials discussed in this manuscript apart from those disclosed.
Figures





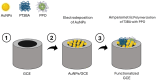
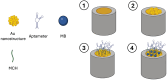

References
-
- Bryant B., Knights K. Pharmacology for Health Professionals Ebook. Elsevier Health Sciences; Philadelphia, PA, USA: 2014.
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

