Dietary Palmitic Acid Drives a Palmitoyltransferase ZDHHC15-YAP Feedback Loop Promoting Tumor Metastasis
- PMID: 39686664
- PMCID: PMC11809420
- DOI: 10.1002/advs.202409883
Dietary Palmitic Acid Drives a Palmitoyltransferase ZDHHC15-YAP Feedback Loop Promoting Tumor Metastasis
Abstract
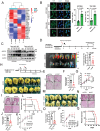
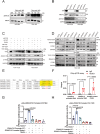
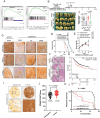
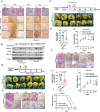
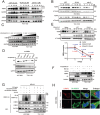
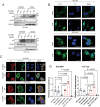
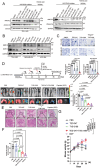
Elevated uptake of saturated fatty acid palmitic acid (PA) is associated with tumor metastasis; however, the precise mechanisms remain partially understood, hindering the development of therapy for PA-driven tumor metastasis. The Hippo-Yes-associated protein (Hippo/YAP) pathway is implicated in cancer progression. Here it is shown that a high-palm oil diet potentiates tumor metastasis in murine xenografts in part through YAP. It is found that the palmitoyltransferase ZDHHC15 is a YAP-regulated gene that forms a feedback loop with YAP. Notably, PA drives the ZDHHC15-YAP feedback loop, thus enforces YAP signaling, and hence promotes tumor metastasis in murine xenografts. In addition, it is shown that ZDHHC15 associates with Kidney and brain protein (KIBRA, also known as WW- and C2 domain-containing protein 1, WWC1), an upstream component of Hippo signaling, and mediates its palmitoylation. KIBRA palmitoylation leads to its degradation and regulates its subcellular localization and activity toward the Hippo/YAP pathway. Moreover, PA enhances KIBRA palmitoylation and degradation. It is further shown that combinatorial targeting of YAP and fatty acid synthesis exhibits augmented effects against metastasis formation in mice fed with a Palm diet. Collectively, these findings uncover a ZDHHC15-YAP feedback loop as a previously unrecognized mechanism underlying PA-promoted tumor metastasis and support targeting YAP and fatty acid synthesis as potential therapeutic targets in PA-driven tumor metastasis.
Keywords: S‐palmitoylations; YAP; ZDHHC15; palmitic acids; tumor metastasis.
© 2024 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
