Time-of-day control of mitochondria regulates NLRP3 inflammasome activation in macrophages
- PMID: 39686706
- PMCID: PMC11669068
- DOI: 10.1096/fj.202400508RR
Time-of-day control of mitochondria regulates NLRP3 inflammasome activation in macrophages
Abstract
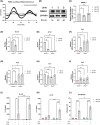
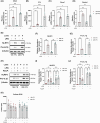
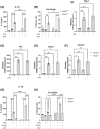
Macrophages are innate immune cells that orchestrate the process of inflammation, which varies across time of day. This ensures appropriate biological timing of the immune response with the external environment. The NLRP3 inflammasome mediates IL-1-family cytokine release via pyroptosis. Mitochondria play a multifaceted role regulating NLRP3 inflammasome activity. Mitochondria exhibit distinct metabolic changes across time of day, which are influenced by clock genes. However, whether the macrophage clock regulates the NLRP3 inflammasome via mitochondrial control remains unclear. We find heightened mitochondrial membrane potential (Δψm) and enhanced NLRP3 inflammasome activation from peritoneal exudate cells (PECs) isolated at circadian time (CT) 12 compared to CT 0. In vitro time-of-day synchronization of bone-marrow derived macrophages (BMDMs) induced time-dependent differences in NLRP3 inflammasome activation. Myeloid-specific Bmal1-deletion enhanced NLRP3 inflammasome activity in PECs at CT0 and in unsynchronized BMDMs compared to controls. Pharmacologically disrupting Δψm in synchronized cells reduced NLRP3 inflammasome activation to comparable levels, and the same occurred with Bmal1-deletion. These results further demonstrate circadian clock timing of the NLRP3 inflammasome, which is dependent on mitochondrial function and driven through the circadian gene Bmal1.
Keywords: NLRP3 inflammasome; circadian rhythms; macrophages; mitochondria; pyroptosis.
© 2024 Federation of American Societies for Experimental Biology.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

