Efficacy and Safety of Atezolizumab plus Bevacizumab versus Sorafenib in Hepatocellular Carcinoma with Main Trunk and/or Contralateral Portal Vein Invasion in IMbrave150
- PMID: 39687036
- PMCID: PMC11649257
- DOI: 10.1159/000539897
Efficacy and Safety of Atezolizumab plus Bevacizumab versus Sorafenib in Hepatocellular Carcinoma with Main Trunk and/or Contralateral Portal Vein Invasion in IMbrave150
Abstract
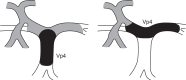
Introduction: Atezolizumab plus bevacizumab significantly improved overall survival (OS) and progression-free survival (PFS) versus sorafenib in patients with unresectable hepatocellular carcinoma (HCC) in IMbrave150. Efficacy and safety in patient subpopulations with Vp4 portal vein tumor thrombosis (PVTT) and other high-risk prognostic factors are reported.
Methods: IMbrave150 was a global, randomized (2:1), open-label, phase 3 study in systemic treatment-naive patients with unresectable HCC; OS and PFS were co-primary endpoints. Exploratory analyses compared the efficacy and safety of atezolizumab 1,200 mg plus bevacizumab 15 mg/kg every 3 weeks versus sorafenib 400 mg twice daily in patients (i) with and without Vp4 PVTT alone and (ii) with and without high-risk prognostic factors.
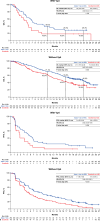
Results: In patients with Vp4 PVTT, median OS was 7.6 months (95% CI: 6.0-13.9) with atezolizumab plus bevacizumab (n = 48) and 5.5 months (95% CI: 3.4-6.7) with sorafenib (n = 25; HR 0.62 [95% CI: 0.34-1.11]; descriptive p = 0.104). Median PFS in the respective arms was 5.4 months (95% CI: 3.6-6.9) and 2.8 months (95% CI: 1.5-5.3; HR 0.62 [95% CI: 0.35-1.09]; descriptive p = 0.094). In patients without Vp4, median OS was 21.1 months (95% CI: 18.0-24.6) with atezolizumab plus bevacizumab (n = 288) and 15.4 months (95% CI: 12.6-18.6) with sorafenib (n = 140; HR 0.67 [95% CI: 0.51-0.88]; descriptive p = 0.003). Median PFS in the respective arms was 7.1 months (95% CI: 6.1-9.6) and 4.7 months (95% CI: 4.2-6.1; HR 0.64 [95% CI: 0.51-0.81]; descriptive p < 0.001). The high-risk versus non-high-risk populations had similar outcome patterns. In the respective treatment arms, grade ≥3 treatment-related adverse events occurred in 43% and 48% of patients with Vp4 and 46% and 47% of patients without Vp4.
Conclusion: Regardless of VP4 PVTT or other high-risk features of unresectable HCC, which have often resulted in exclusion from other front-line trials, patients benefited from atezolizumab and bevacizumab versus sorafenib.
Keywords: High-risk prognostic factors; Immunotherapy; PD-L1 inhibitor; Portal vein tumor thrombosis; Vp4.
© 2024 The Author(s). Published by S. Karger AG, Basel.
Conflict of interest statement
Richard S. Finn reports receiving consulting fees from AstraZeneca, Bayer, CStone Pharmaceuticals, Eisai, Eli Lilly, Exelixis, F. Hoffmann-La Roche Hengrui, Merck, and Pfizer; research funding to institution from Adaptimmune, Bristol Myers Squibb, Eisai, Eli Lilly, Merck, Pfizer, and F. Hoffmann-La Roche Ltd; and is an Editorial Board Member of Liver Cancer. Peter R. Galle reports receiving consulting fees from Adaptimmune, AstraZeneca, Bayer, Boston Scientific, Bristol Myers Squibb, Eisai, Eli Lilly, F. Hoffmann-La Roche Ltd., Guerbet, Ipsen, Merck Sharp and Dohme, and Sirtex Medical; honoraria from Adaptimmune, AstraZeneca, Bayer, Boston Scientific, Bristol Myers Squibb, Eisai, Eli Lilly, F. Hoffmann-La Roche Ltd., Guerbet, Ipsen, Merck Sharp and Dohme, and Sirtex Medical; advisory fees from Adaptimmune, AstraZeneca, Bayer, Boston Scientific, Bristol Myers Squibb, Eisai, Eli Lilly, F. Hoffmann-La Roche Ltd., Guerbet, Ipsen, Merck Sharp and Dohme, and Sirtex Medical; and research funding to institution from Bayer and F. Hoffmann-La Roche Ltd. Michel Ducreux reports receiving honoraria, consulting fees, or advisory fees to self from Amgen, AstraZeneca, Bayer, Eli Lilly, F. Hoffmann-La Roche Ltd., Ipsen, Merck Serono, Pierre Fabre, and Servier; travel support from Bayer, Eli Lilly, F. Hoffmann-La Roche Ltd., Ipsen, Merck Sharp & Dohme, and Servier; speaker bureau participation for Amgen, Bayer, Eli Lilly, F. Hoffmann-La Roche Ltd., Ipsen, and Merck Serono; and research funding to institution from Bayer and F. Hoffmann-La Roche Ltd. Ann-Lii Cheng reports receiving research funding to institution from F. Hoffmann-La Roche Ltd. Alan Nicholas is an employee and stockholder of Roche/Genentech. Philippe Merle has had a consulting or advisory role for Bayer, Ipsen, Lilly, Eisai, AstraZeneca, Bristol Myers Squibb, and MSD; received institutional research funding from Ipsen; and travel and accommodation expenses from Bayer and Ipsen. Riad Salem is a consultant for Boston Scientific, Eisai, Genentech, Cook, Sirtex and AstraZeneca. Daneng Li has received honoraria and advisory/consultancy fees from AstraZeneca, Ipsen, Eisai, Exelixis, Coherus, Genentech, QED, Merck, Adagene, Delcath, Servier, Sumitomo, Transthera, and TerSera; and received institutional research funding from Brooklyn Immunotherapeutics and AstraZeneca. Valeriy Breder has received advisory/consultancy fees from F. Hoffmann-La Roche, MSD, Eisai, Bristol Myers Squibb, and Ipsen; and travel and accommodation expenses from F. Hoffmann-La Roche, MSD, Eisai, Bristol Myers Squibb, and Bayer. Ning Ma is an employee and stockholder of Roche/Genentech. Sairy Hernandez is an employee and stockholder of Roche/Genentech.
Figures
References
-
- Qiu G, Xie K, Jin Z, Jiang C, Liu H, Wan H, et al. . The multidisciplinary management of hepatocellular carcinoma with portal vein tumor thrombus. Biosci Trends. 2021;15(3):148–54. - PubMed
-
- Liver Cancer Study Group of Japan . The general rules for the clinical and pathological study of primary liver cancer. Jpn J Surg. 1989;19(1):98–129. - PubMed
-
- Mähringer-Kunz A, Steinle V, Düber C, Weinmann A, Koch S, Schmidtmann I, et al. . Extent of portal vein tumour thrombosis in patients with hepatocellular carcinoma: the more, the worse? Liver Int. 2019;39(2):324–31. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

