An attempted oxidative coupling approach to the scholarinine A framework
- PMID: 39687046
- PMCID: PMC11649313
- DOI: 10.1016/j.tetlet.2024.154980
An attempted oxidative coupling approach to the scholarinine A framework
Abstract
In this manuscript, an oxidative carbon-carbon bond forming reaction to construct the framework of alkaloids such as scholarinine A is explored using a constrained substrate. Instead of the desired carbon-carbon bond formation between an indole C3 position and a malonate group, a competing carbon-nitrogen bond between the malonate and indole C3 position was observed to form. This work adds to the growing body of substrates for oxidative carbon-carbon bond formation and importantly, demonstrates that these reactions are challenging for some conformationally constrained substrates.
Keywords: Alkaloids’ akuammiline; Indole; Oxidative coupling; Scholarinine A; Total synthesis.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
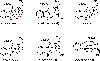




References
-
- Zhan R, Du S-Z, Duan Z-H, Nian Y, Chen Y-G, Scholarinine a, a N3 T-type caged-monoterpene indole alkaloid as Cav3.1 T-type Calcium Channel inhibitor from alstonia scholaris, Tetrahedron Lett. 61 (2020) 151354.
-
- Sparks TC, Lorsbach BA, Perspectives on the agrochemical industry and agrochemical discovery, Pest Manag. Sci. 73 (2017) 672–677. - PubMed
-
- King GF, Escoubas P, Nicholson GM, Peptide toxins that selectively target insect nav and cav channels, Channels 2 (2008) 100–116. - PubMed
-
- Krishnan P, Mai C-W, Yong K-T, Low Y-Y, Lim K-H, Alstobrogaline, an unusual pentacyclic monoterpenoid indole alkaloid with aldimine and aldimine-N-oxide moieties from alstonia scholaris, Tetrahedron Lett. 60 (2019) 789–791;
- Wang Z, Xiao Y, Wu S, Chen J, Li A, Tatsis EC, Deciphering and reprogramming the cyclization regioselectivity in bifurcation of indole alkaloid biosynthesis, Chem. Sci. 13 (2022) 12389–12395. - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

