Circulating tumor DNA-guided treatment decision in metastatic castration-resistant prostate cancer patients: a cost-effectiveness analysis
- PMID: 39687053
- PMCID: PMC11648017
- DOI: 10.1177/17588359241305084
Circulating tumor DNA-guided treatment decision in metastatic castration-resistant prostate cancer patients: a cost-effectiveness analysis
Abstract
Background: The androgen receptor pathway inhibitors (ARPI), abiraterone acetate and enzalutamide, are commonly used in first-line treatment of patients with metastatic castration-resistant prostate cancer (mCRPC). However, early resistance to ARPI treatment occurs frequently. Traditionally, the response is evaluated 3-6 months after the start of treatment. However, recent findings indicate that by detecting circulating tumor DNA (ctDNA) at baseline and 4 weeks after ARPI treatment initiation, patients with a nondurable response can be identified after 4 weeks of treatment, enabling an early switch to alternative treatments.
Objective: This study aims to evaluate the cost-effectiveness of ctDNA-guided treatment switch after 4 weeks of ARPI therapy in mCRPC patients compared to standard of care.
Design: A cost-effectiveness analysis.
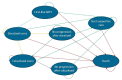
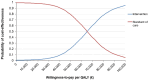
Methods: A cost-effectiveness analysis was conducted by creating a Markov state transition model to simulate progression, mortality, and treatment costs over a 5-year time horizon comparing ctDNA-guided care versus standard of care. The outcomes measured were incremental treatment costs per life-years and quality-adjusted life-years (QALYs) gained.
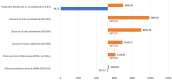
Results: The analysis showed an incremental cost-effectiveness ratio of €65,400.86 per QALY gained and an incremental net monetary benefit of €2716.62. Thereby, the use of ctDNA-guided treatment was cost-effective in comparison to standard care in 74% of the simulations using a willingness-to-pay threshold of €80,000 per QALY gained.
Conclusion: Our study demonstrated the cost-effectiveness of using a ctDNA-guided early therapy switch in non-responders after only 4 weeks of first-line ARPI therapy in mCRPC patients. This paves the way for implementing ctDNA-guided treatment decisions in clinical practice.
Keywords: androgen receptor pathway inhibitors; biomarkers; chemotherapy; circulating tumor DNA (ctDNA); cost-effectiveness analysis; health services research; hormone therapy; liquid biopsy; prostate cancer.
© The Author(s), 2024.
Conflict of interest statement
N.M. received consultancy fees from Janssen-Cilag, Bayer, Astellas Pharma, AstraZeneca, Pfizer, and MSD and research funding (all paid to the institute) from Janssen-Cilag, Astellas Pharma, AstraZeneca/Merck, and Bristol Myers Squibb Foundation. I.M.v.O. received consultancy fees (all paid to the institute) from Astellas, Bayer, Janssen, Astrazeneca/MSD, Pfizer, and Novartis. N.P.v.E. has received research grants (all paid to the institute) from Astellas and Ipsen. W.K., S.J.E.B., C.J.P.O.t.H., and E.B. declare no potential conflicts of interest.
Figures

References
-
- Fizazi K, Gillessen S; ESMO Guidelines Committee. Updated treatment recommendations for prostate cancer from the ESMO Clinical Practice Guideline considering treatment intensification and use of novel systemic agents. Ann Oncol 2023; 34: 557–563. - PubMed
-
- Parker C, Castro E, Fizazi K, et al. Prostate cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2020; 31: 1119–1134. - PubMed
-
- Ryan CJ, Smith MR, Fizazi K, et al. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol 2015; 16: 152–160. - PubMed
-
- Armstrong AJ, Lin P, Tombal B, et al. Five-year survival prediction and safety outcomes with enzalutamide in men with chemotherapy-naive metastatic castration-resistant prostate cancer from the PREVAIL trial. Eur Urol 2020; 78: 347–357. - PubMed
LinkOut - more resources
Full Text Sources

