Effects of nintedanib on circulating biomarkers of idiopathic pulmonary fibrosis
- PMID: 39687396
- PMCID: PMC11647937
- DOI: 10.1183/23120541.00558-2023
Effects of nintedanib on circulating biomarkers of idiopathic pulmonary fibrosis
Abstract
Background: Biomarkers that change in response to nintedanib in subjects with idiopathic pulmonary fibrosis (IPF) would be valuable. We investigated the effects of nintedanib on circulating biomarkers in subjects with IPF in the INMARK trial.
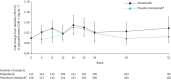
Methods: Subjects with IPF were randomised 1:2 to receive nintedanib 150 mg twice daily or placebo for 12 weeks, after which all patients received open-label nintedanib for 40 weeks. Fold changes in adjusted mean levels of circulating biomarkers were analysed using a linear mixed model for repeated measures.
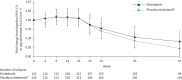
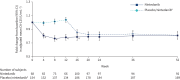
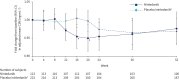
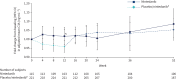
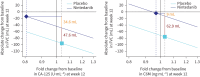
Results: 346 subjects were treated (116 randomised to nintedanib, 230 to placebo). Surfactant protein D (SP-D) and cancer antigen 125 (CA-125), markers of epithelial injury, decreased in subjects treated with nintedanib versus placebo. Fold changes from baseline in SP-D at week 12 corresponded to a 4% decrease and 3% increase in the nintedanib and placebo groups, respectively (ratio 0.94, 95% CI 0.89-0.99; p=0.024). Fold changes in CA-125 at week 12 corresponded to a 22% decrease and 4% increase in the nintedanib and placebo groups, respectively (ratio 0.75, 95% CI 0.71-0.81; p<0.0001). A mediation analysis suggested that 42.1% of the effect of nintedanib on change in forced vital capacity over 12 weeks was attributable to the change in CA-125. A small increase in C3A (collagen 3 degraded by ADAMTS-1/4/8) and a small decrease in C3M (collagen 3 degraded by matrix metalloproteinase-9), markers of extracellular matrix turnover, were observed in subjects treated with nintedanib versus placebo.
Conclusions: Effects of nintedanib on circulating markers of epithelial dysfunction and collagen degradation, most notably CA-125, were observed in patients with IPF.
Copyright ©The authors 2024.
Conflict of interest statement
Conflict of interest: R.G. Jenkins has received grants from AstraZeneca, Biogen, Galecto, GlaxoSmithKline, Pliant and RedX; consulting fees from Bristol Myers Squibb, Daewoong Pliant, RedX, Resolution Therapeutics and Veracyte; payment for presentations from AstraZeneca, Chiesi, patientMpower and Roche; has served on a Data Safety Monitoring Board or Advisory Board for Boehringer Ingelheim, Galapagos and Vicore; and has a leadership role with Action for Pulmonary Fibrosis and NuMedii. V. Cottin reports grants from Boehringer Ingelheim; consulting fees from Boehringer Ingelheim, Celgene/Bristol Myers Squibb, CSL Behring, Ferrer, GlaxoSmithKline, Pliant, PureTech, RedX, Roche, Sanofi and Shionogi; payment for presentations and support for attending meetings from Boehringer Ingelheim, Ferrer and Roche; and has served on a Data Safety Monitoring Board or Advisory Board for Celgene/Bristol Myers Squibb, FibroGen, Galapagos, Galecto and Roche. Y. Nishioka reports grants and payment for presentations from Boehringer Ingelheim. I. Noth reports grants from Veracyte; royalties from UpToDate; consulting fees from Boehringer Ingelheim, Genentech and Sanofi; patents for a gene signature predictor of forced vital capacity and for PCSK6 (pending); and has served on a Data Safety Monitoring Board for Yale University. E.S. White, C. Ittrich, C. Diefenbach and K.B. Rohr are employees of Boehringer Ingelheim. M. Selman was a member of an Adjudication Committee for Celgene. T.M. Maher reports consulting fees from AstraZeneca, Bayer, Blade Therapeutics, Boehringer Ingelheim, Bristol Myers Squibb, Galapagos, Galecto, GlaxoSmithKline, IQVIA, Pliant, Respivant, Roche/Genentech, Theravance and Veracyte; and payment for presentations from Boehringer Ingelheim and Roche/Genentech.
Figures

References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
