Anesthesia-induced Developmental Neurotoxicity in Pediatric Population
- PMID: 39687550
- PMCID: PMC11649317
- DOI: 10.26502/jsr.10020400
Anesthesia-induced Developmental Neurotoxicity in Pediatric Population
Abstract
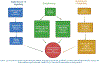
Anesthetics and sedatives may cause long-term negative neurocognitive consequences in children. Many clinical reports on this subject have had a profound impact on the field of clinical pediatric anesthesiology. Findings from animal models suggest that early exposure to anesthesia might cause neurocognitive impairment and apoptotic cell death in the brain. Even though the findings from the experimental animals cannot be directly translated to the use of anesthesia in pediatric population due to many variable factors, parents and government regulatory bodies have become sensitive and attentive to the potential adverse effects of anesthesia in children. Multiple epidemiological investigations in human have added to the growing body of evidence showing neurological impairment and cognitive decline after early anesthetic exposure. This is supported by several outcome indicators, including validated neuropsychologic testing, educational interventions for neurodevelopmental or behavioral disorders, and academic performance or school readiness. These outcomes have been evaluated in clinical studies involving children who have been subjected to general anesthesia. The primary goal of this article is to critically review the clinical findings, perform systematic analyses of the evidence, discuss potential underlying mechanisms of neurotoxicity, the pathophysiology of anesthesia-induced developmental neurotoxicity involving mitochondria, endoplasmic reticulum, and lysosomes, and the ethical considerations of pediatric anesthesia. Although detailed well-controlled clinical studies are warranted, the evidence so far support that the potential adverse effects of surgical anesthesia to induce neurotoxicity in pediatric population are not exaggerated.
Keywords: Anesthesia-induced developmental neurotoxicity; Apoptotic pathways; Congenital heart disease; Endoplasmic reticulum; GABA agonist; Hypoxia; Lysosomes; Mitochondrial dysfunction; NMDA antagonist; Neuroapoptosis; Neurocognitive impairment; Neurodevelopmental outcomes; Neurogenesis; Neurotoxicity; Pediatric anesthesia; Synaptogenesis.
Conflict of interest statement
Competing interests All authors have read the manuscript and declare no conflict of interest. No writing assistance was utilized in the production of this manuscript.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources
