Protocol for engineering bone organoids from mesenchymal stem cells
- PMID: 39687559
- PMCID: PMC11647664
- DOI: 10.1016/j.bioactmat.2024.11.017
Protocol for engineering bone organoids from mesenchymal stem cells
Abstract
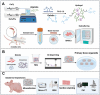
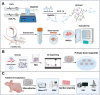
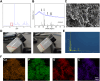
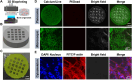
Bone organoids are emerging as powerful tools for studying bone development and related diseases. However, the simplified design of current methods somewhat limits their application potential, as these methods produce single-tissue organoids that fail to replicate the bone microarchitecture or achieve effective mineralization. To address this issue, we propose a three-dimensional (3D) construction strategy for generating mineralized bone structures using bone marrow-derived mesenchymal stem cells (BMSCs). By mixing BMSCs with hydrogel to create a bone matrix-mimicking bioink and employing projection-based light-curing 3D printing technology, we constructed 3D-printed structures, which were then implanted subcutaneously into nude mice, away from the native bone microenvironment. Even without external stimulation, these implants spontaneously formed mineralized bone domains. With long-term culture, these structures gradually matured into fully differentiated bone tissue, completing both mineralization and vascularization. This in vivo bone organoid model offers a novel platform for studying bone development, exploring congenital diseases, testing drugs, and developing therapeutic applications.
Keywords: 3D bioprinting; Bioink; Bone organoids; Mineralization; Vascularization.
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
-
- Wang J., Li X., Wang S., Cui J., Ren X., Su J. Bone-targeted exosomes: strategies and applications. Adv. Healthcare Mater. 2023;12(18) - PubMed
LinkOut - more resources
Full Text Sources

