Perioperative tislelizumab with four cycles of neoadjuvant chemotherapy for resectable locally advanced esophageal squamous cell carcinoma: a phase 2 study
- PMID: 39687611
- PMCID: PMC11647006
- DOI: 10.3389/fimmu.2024.1482005
Perioperative tislelizumab with four cycles of neoadjuvant chemotherapy for resectable locally advanced esophageal squamous cell carcinoma: a phase 2 study
Abstract
Background: The application of neoadjuvant immunotherapy in the treatment of esophageal cancer needs further exploration. This study aimed to investigate the safety and effectiveness of tislelizumab, an anti-PD-1 antibody, combined with chemotherapy as neoadjuvant treatment for locally advanced esophageal squamous cell carcinoma (LA-ESCC).
Methods: In this phase II study, patients with clinical stages of II-IVA (T3-T4 and/or node positive) potentially resectable LA-ESCC were enrolled. Patients received neoadjuvant tislelizumab and chemotherapy every 3 weeks for 4 cycles before surgery and adjuvant tislelizumab for 9 months. The primary endpoint was pathological complete response (pCR) rate. Secondary endpoints included R0 resection, disease free survival (DFS), adverse events (AE), and biomarkers for predicting efficacy.
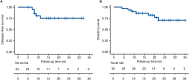
Results: The study included 30 patients. 25 patients completed neoadjuvant chemoimmunotherapy and underwent surgery, 96% with R0 resection. The pCR and MPR rate was 44% and 52%. The 6-month and 1-year DFS rate was 100% and 75.3%. 43.3% patients experienced severe (grade 3-4) treatment-related adverse events (TRAEs) and 5 patients developed severe immune-related adverse events (irAEs). Further exploration found that a group of peripheral lymphocyte subsets increased significantly after 2 cycles of neoadjuvant therapy in patients who achieved pCR, suggesting the importance of dynamic monitoring of circulating lymphocyte.
Conclusions: The combination of perioperative tislelizumab and neoadjuvant chemotherapy has achieved an encouraging pCR rate and demonstrated a manageable safety profile in patients with potentially resectable ESCC.
Clinical trial registration: https://www.chictr.org.cn/, identifier ChiCTR2100043772.
Keywords: esophageal squamous cell carcinoma; immunotherapy; neoadjuvant chemotherapy; perioperative treatment; tislelizumab.
Copyright © 2024 Zhou, Hua, Ge, Wang, Wang, He, Zhao, Yu, Yan, Zhao, Li and Bai.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Perioperative the BTLA inhibitor (tifcemalimab) combined with toripalimab and chemotherapy for resectable locally advanced thoracic esophageal squamous cell carcinoma trial (BT-NICE trial): a prospective, single-arm, exploratory study.Front Immunol. 2025 Apr 10;16:1542877. doi: 10.3389/fimmu.2025.1542877. eCollection 2025. Front Immunol. 2025. PMID: 40276504 Free PMC article.
-
Perioperative tislelizumab plus chemotherapy for locally advanced resectable thoracic esophageal squamous cell carcinoma trial: a prospective single-arm, phase II study (PILOT trial).BMC Cancer. 2023 Dec 15;23(1):1237. doi: 10.1186/s12885-023-11747-9. BMC Cancer. 2023. PMID: 38102553 Free PMC article. Clinical Trial.
-
Mid-term follow-up results of neoadjuvant sintilimab combined with chemotherapy for locally advanced resectable esophageal squamous cell carcinoma.Front Immunol. 2024 Dec 4;15:1453176. doi: 10.3389/fimmu.2024.1453176. eCollection 2024. Front Immunol. 2024. PMID: 39697347 Free PMC article.
-
Efficacy and Safety of Neoadjuvant Chemoimmunotherapy in Resectable Esophageal Squamous Cell Carcinoma: A Meta-analysis.Ann Surg Oncol. 2023 Mar;30(3):1597-1613. doi: 10.1245/s10434-022-12752-1. Epub 2022 Nov 15. Ann Surg Oncol. 2023. PMID: 36380254 Review.
-
Efficacy and safety of neoadjuvant immunotherapy combined with chemotherapy for stage II-IVa esophageal cancer: a network meta-analysis.Syst Rev. 2025 Jan 27;14(1):26. doi: 10.1186/s13643-025-02765-8. Syst Rev. 2025. PMID: 39871293 Free PMC article.
Cited by
-
Neoadjuvant immunochemotherapy in locally advanced esophageal squamous cell carcinoma: a retrospective study with 2-year survival analysis.J Cancer Res Clin Oncol. 2025 Jul 17;151(7):217. doi: 10.1007/s00432-025-06263-1. J Cancer Res Clin Oncol. 2025. PMID: 40676290 Free PMC article.
References
-
- Kato K, Ito Y, Daiko H, Ozawa S, Ogata T, Hara H, et al. . A randomized controlled phase III trial comparing two chemotherapy regimen and chemoradiotherapy regimen as neoadjuvant treatment for locally advanced esophageal cancer, JCOG1109 NExT study. J Clin Oncol. (2022) 40:238–8. doi: 10.1200/JCO.2022.40.4_suppl.238 - DOI
-
- Shapiro J, van Lanschot JJB, Hulshof MCCM, van Hagen P, van Berge Henegouwen MI, Wijnhoven BPL, et al. . Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol. (2015) 16:1090–8. doi: 10.1016/S1470-2045(15)00040-6 - DOI - PubMed
-
- Hall PS, Swinson D, Cairns DA, Waters JS, Petty R, Allmark C, et al. . Efficacy of reduced-intensity chemotherapy with oxaliplatin and capecitabine on quality of life and cancer control among older and frail patients with advanced gastroesophageal cancer: the GO2 phase 3 randomized clinical trial. JAMA Oncol. (2021) 7:869–77. doi: 10.1001/jamaoncol.2021.0848 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

