Hyper-fractionated radiotherapy as a bridging strategy to enhance CAR-T efficacy by regulating T-cell co-stimulatory molecules in relapsed/refractory diffuse large B-cell lymphoma
- PMID: 39687615
- PMCID: PMC11646978
- DOI: 10.3389/fimmu.2024.1481080
Hyper-fractionated radiotherapy as a bridging strategy to enhance CAR-T efficacy by regulating T-cell co-stimulatory molecules in relapsed/refractory diffuse large B-cell lymphoma
Abstract
Background: Bridging therapy can prevent patients from disease progression while waiting for CAR-T cell preparation. Hyper-fractionated radiotherapy can achieve an effective target dose within a short period, minimize radiation damage, and may modify immune environment compared to conventional radiotherapy.
Aims: This study aims to investigate the efficacy and safety of bridging hyper-fractionated radiotherapy in combination with CAR-T therapy for relapsed/refractory diffuse large B-cell lymphoma. The potential mechanisms were explored.
Methods: This is a prospective pilot study. After T-cell collection, the patients underwent hyper-fractionated radiotherapy at lesion sites with 1.5 Gy twice daily for 10 days before CAR-T cell infusion. Peripheral blood immune cell subsets and quantitative serum proteomics were assessed before radiotherapy and after radiotherapy before CAR-T cell infusion.
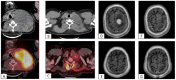
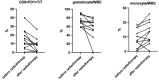
Results: A total of 13 patients have been enrolled. The median follow-up time was 6 (3-24) months after CAR-T infusion. At 3-month follow-up, 9/13(69%) patients had CR, 1/13(8%) patient had PR, 1/13(8%) patient remained SD, and 2/13(15%) patients died of disease progression. The local recurrence rate was 1/13(8%). Seven patients have been followed up for more than 6 months, and they remain in CR. The median PFS and OS were not reached. No grade 3-4 CRS or ICANS were reported. After hyper-fractionated radiotherapy, peripheral PD1+CD8+T/T ratio significantly decreased while quantitative serum proteomics profiling showed a decrease in sCD28.
Conclusion: Hyper-fractionated radiotherapy can rapidly control tumor progression sites without delaying the infusion time. This approach can improve the ORR and does not increase the incidence of CRS and ICANS. The mechanism may be related to the regulation of T-cell co-stimulatory molecules, which demands further exploration.
Keywords: CAR-T; DLBCL; T-cell co-stimulatory molecules; bridging therapy; hyper-fractionated radiotherapy.
Copyright © 2024 Ruan, Zhou, Zhang, Zhao, Wei, Hu, Zhang, Hou and Zhang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Phase I Trial of Fourth-Generation Anti-CD19 Chimeric Antigen Receptor T Cells Against Relapsed or Refractory B Cell Non-Hodgkin Lymphomas.Front Immunol. 2020 Nov 27;11:564099. doi: 10.3389/fimmu.2020.564099. eCollection 2020. Front Immunol. 2020. PMID: 33329526 Free PMC article. Clinical Trial.
-
Phase II trial of co-administration of CD19- and CD20-targeted chimeric antigen receptor T cells for relapsed and refractory diffuse large B cell lymphoma.Cancer Med. 2020 Aug;9(16):5827-5838. doi: 10.1002/cam4.3259. Epub 2020 Jul 1. Cancer Med. 2020. PMID: 32608579 Free PMC article. Clinical Trial.
-
[18F]FDG PET/CT for prognosis and toxicity prediction of diffuse large B-cell lymphoma patients with chimeric antigen receptor T-cell therapy.Eur J Nucl Med Mol Imaging. 2024 Jul;51(8):2308-2319. doi: 10.1007/s00259-024-06667-0. Epub 2024 Mar 12. Eur J Nucl Med Mol Imaging. 2024. PMID: 38467921
-
The Role of Radiotherapy in Lymphoma Patients Undergoing CAR T Therapy: Past, Present, and Future.Semin Radiat Oncol. 2025 Jan;35(1):99-109. doi: 10.1016/j.semradonc.2024.10.005. Semin Radiat Oncol. 2025. PMID: 39672646 Review.
-
Assessing the role of radiotherapy in patients with refractory or relapsed high-grade B-cell lymphomas treated with CAR T-cell therapy.Radiother Oncol. 2022 Oct;175:65-72. doi: 10.1016/j.radonc.2022.08.007. Epub 2022 Aug 8. Radiother Oncol. 2022. PMID: 35952976 Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

