Entamoeba histolytica-induced NETs are highly cytotoxic on hepatic and colonic cells due to serine proteases and myeloperoxidase activities
- PMID: 39687618
- PMCID: PMC11646992
- DOI: 10.3389/fimmu.2024.1493946
Entamoeba histolytica-induced NETs are highly cytotoxic on hepatic and colonic cells due to serine proteases and myeloperoxidase activities
Abstract
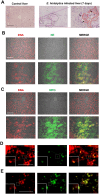
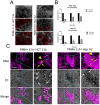
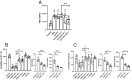
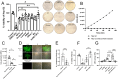
During intestinal and liver invasion by the protozoan parasite Entamoeba histolytica, extensive tissue destruction linked to large neutrophil infiltrates is observed. It has been proposed that microbicidal components of neutrophils are responsible for the damage, however, the mechanism by which they are released and act in the extracellular space remains unknown. In previous studies, we have shown that E. histolytica trophozoites induce NET formation, leading to the release of neutrophil granule content into extruded DNA. In this work, we evaluate the possible participation of NETs in the development of amoeba-associated pathology and analyze the contribution of anti-microbial components of the associated granules. E. histolytica-induced NETs were isolated and their effect on the viability and integrity of HCT 116 colonic and Hep G2 liver cultures were evaluated. The results showed that simple incubation of cell monolayers with purified NETs for 24 h resulted in cell detachment and death in a dose-dependent manner. The effect was thermolabile and correlated with the amount of DNA and protein present in NETs. Pretreatment of NETs with specific inhibitors of some microbicidal components suggested that serine proteases, are mostly responsible for the damage caused by NETs on HCT 116 cells, while the MPO activity was the most related to Hep G2 cells damage. Our study also points to a very important role of DNA as a scaffold for the activity of these proteins. We show evidence of the development of NETs in amoebic liver abscesses in hamsters as a preamble to evaluate their participation in tissue damage. In conclusion, these studies demonstrate that amoebic-induced NETs have potent cytotoxic effects on target cells and, therefore, may be responsible for the intense damage associated with tissue invasion by this parasite.
Keywords: Entamoeba histolytica; HCT 116; Hep G2; NETs; cell damage; neutrophils.
Copyright © 2024 Jorge-Rosas, Díaz-Godínez, García-Aguirre, Martínez-Calvillo and Carrero.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Bottazzi ME, Dumonteil E, Valenzuela JG, Betancourt-Cravioto M, Tapia-Conyer R, Hotez PJ. Bridging the innovation gap for neglected tropical diseases in Mexico: capacity building for the development of a new generation of antipoverty vaccines. Bol Med Hosp Infant Mex. (2011) 2:138–46.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

