A Novel Intersection: Cytomegalovirus Gastritis Following Cemiplimab and Talimogene Laherparepvec in a Patient With Advanced Cutaneous Squamous Cell Carcinoma
- PMID: 39687659
- PMCID: PMC11646813
- DOI: 10.1002/ccr3.9632
A Novel Intersection: Cytomegalovirus Gastritis Following Cemiplimab and Talimogene Laherparepvec in a Patient With Advanced Cutaneous Squamous Cell Carcinoma
Abstract
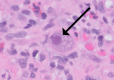
Cytomegalovirus (CMV) reactivation is a rare complication in patients treated with immune checkpoint inhibitors (ICIs), typically occurring after immunosuppressive therapy for immune-related adverse events (irAEs). Here, we report a unique case of severe CMV gastritis in a patient receiving cemiplimab, an anti-PD-1 antibody, and talimogene laherparepvec (T-VEC), an oncolytic virus, without prior irAEs or immunosuppressive treatment. A 63-year-old man with advanced cutaneous squamous cell carcinoma received cemiplimab for one year and a single T-VEC injection for recurrent disease. He presented with progressive dyspepsia, significant weight loss, and malnutrition requiring total parenteral nutrition. Endoscopy revealed extensive gastric ulceration, and biopsies confirmed CMV gastritis. Initial treatment with intravenous ganciclovir improved his viral load but provided minimal symptomatic relief. After six weeks of therapy, biopsies showed resolution of CMV infection, and the patient transitioned to oral valganciclovir for prophylaxis while resuming cancer treatment. This case highlights the potential for CMV reactivation in patients undergoing ICI therapy, even without prior immunosuppression or irAEs. Notably, the concurrent use of T-VEC raises questions about the interplay between oncolytic viruses, ICIs, and immune modulation. Although T-VEC is not known to directly cause CMV reactivation, its role in amplifying immune responses warrants further investigation. As ICIs and oncolytic viruses become increasingly integral in oncology, clinicians must remain vigilant for rare infectious complications like CMV reactivation. Further research is needed to elucidate mechanisms, identify risk factors, and optimize management strategies for these events.
Keywords: cemiplimab; cytomegalovirus; immunotherapy; talimogene laherparepvec.
© 2024 The Author(s). Clinical Case Reports published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Del Castillo M., Romero F. A., Argüello E., Kyi C., Postow M. A., and Redelman‐Sidi G., “The Spectrum of Serious Infections Among Patients Receiving Immune Checkpoint Blockade for the Treatment of Melanoma,” Clinical Infectious Diseases 63, no. 11 (2016): 1490–1493, 10.1093/cid/ciw539. - DOI - PMC - PubMed
-
- Tay K. H., Slavin M. A., Thursky K. A., et al., “Cytomegalovirus DNAemia and Disease: Current‐Era Epidemiology, Clinical Characteristics and Outcomes in Cancer Patients Other Than Allogeneic Haemopoietic Transplantation,” Internal Medicine Journal 52, no. 10 (2022): 1759–1767, 10.1111/imj.15496. - DOI - PubMed
-
- Mager L., Gardeen S., Carr D. R., and Shahwan K. T., “Cemiplimab for the Treatment of Advanced Cutaneous Squamous Cell Carcinoma: Appropriate Patient Selection and Perspectives,” Clinical, Cosmetic and Investigational Dermatology 9, no. 16 (2023): 2135–2142, 10.2147/CCID.S381471. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

