Beyond-local neural information processing in neuronal networks
- PMID: 39687759
- PMCID: PMC11647244
- DOI: 10.1016/j.csbj.2024.10.040
Beyond-local neural information processing in neuronal networks
Abstract
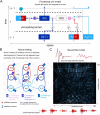
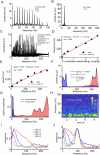
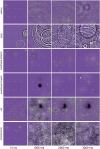
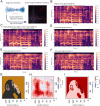
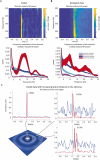
While there is much knowledge about local neuronal circuitry, considerably less is known about how neuronal input is integrated and combined across neuronal networks to encode higher order brain functions. One challenge lies in the large number of complex neural interactions. Neural networks use oscillating activity for information exchange between distributed nodes. To better understand building principles underlying the observation of synchronized oscillatory activity in a large-scale network, we developed a reductionistic neuronal network model. Fundamental building principles are laterally and temporally interconnected virtual nodes (microcircuits), wherein each node was modeled as a local oscillator. By this building principle, the neuronal network model can integrate information in time and space. The simulation gives rise to a wave interference pattern that spreads over all simulated columns in form of a travelling wave. The model design stabilizes states of efficient information processing across all participating neuronal equivalents. Model-specific oscillatory patterns, generated by complex input stimuli, were similar to electrophysiological high-frequency signals that we could confirm in the primate visual cortex during a visual perception task. Important oscillatory model pre-runners, limitations and strength of our reductionistic model are discussed. Our simple scalable model shows unique integration properties and successfully reproduces a variety of biological phenomena such as harmonics, coherence patterns, frequency-speed relationships, and oscillatory activities. We suggest that our scalable model simulates aspects of a basic building principle underlying oscillatory, large-scale integration of information in small and large brains.
Keywords: Columnar architecture; Information integration; Neural network; Neuronal field model; Neuronal oscillations; Parallel computing; Visual perception.
© 2024 The Authors.
Figures


References
-
- Abasolo D., Hornero R., Gomez C., Garcia M., Lopez M. Analysis of EEG background activity in Alzheimer’s disease patients with Lempel-Ziv complexity and central tendency measure. Med Eng Phys. 2006;28(4):315–322. - PubMed
-
- Aguilera M. Scaling behaviour and critical phase transitions in integrated information theory. Entropy. 2019;21:1198.
LinkOut - more resources
Full Text Sources
