Stoichiometry effect on the structure, coordination and anticancer activity of gold(I/III) bisphosphine complexes
- PMID: 39688257
- PMCID: PMC11650976
- DOI: 10.1039/d4dt01663g
Stoichiometry effect on the structure, coordination and anticancer activity of gold(I/III) bisphosphine complexes
Abstract
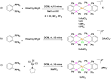
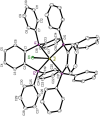
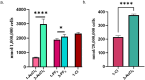
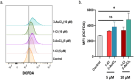
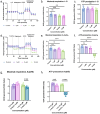
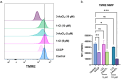
Rationalizing the impact of oxidation states of Au-based complexes on function require synthetic strategies that allow for conserved molecular formula in Au(I) and their Au(III) counterparts. Oftentimes achieving Au(I) and Au(III) coordination complexes with the same ligand system is challenging due to the reactivity and stability of the starting Au(I) or Au(III) starting materials. Thus, attempts to study the impact of oxidation state on biological function has been elusive. We posit that Au complexes with the same ligand framework but different oxidation states will affect complex geometry and hence elicit differences in biological function or mechanism. In this work, we reacted 1,2-bis(diphenylphosphino)benzene with respective Au starting materials in different mole ratios to facilitate the synthesis of structurally distinct Au(I) or Au(III) complexes. Briefly, by reacting two stoichiometric equivalents of HAuCl4·3H2O or AuCl3(tht) with one equivalent of 1,2-bis(diphenylphosphino)benzene, we obtained dicationic bis-[1,2-bis-(diphenylphosphino)benzene]gold(III) chloride whereas an equimolar ratio of HAuCl4·3H2O and 1,2-bis(diphenylphosphino)benzene gave the monocationic bis-[1,2-bis-(diphenylphosphino)benzene]gold(I) complex in moderate yield. The complexes were characterized spectroscopically by HRMS, RP-HPLC-MS, NMR and the purity ascertained by elemental analysis. The 31P NMR showed characteristic singlet peak at ∼22 ppm for the Au(I) complexes and ∼57 ppm for the Au(III) complexes. The structure of the Au(III) complexes was further confirmed by X-ray crystallography as a 5-coordinate Au(III) complex. Although both Au(I) and Au(III) complexes showed promising anticancer activity in MDA-MB-231 (breast cancer) and BT-333 (glioblastoma) cancer cell lines and inhibited maximal mitochondria respiration in MDA-MB-231 cells, the Au(III) complexes further induce ROS accumulation and facilitate depolarization of the mitochondria membrane potential in MDA-MB-231 cells. Taken together, the synthetic approach provides a way to elucidate the effect of Au(I)/Au(III) oxidation states on structure, activity, and potential mechanism with respect to the same ligand.
Conflict of interest statement
The authors declare the following competing financial interest(s): S. G. A. has patents pending to the University of Kentucky Research Foundation.
Figures

References
-
- Kopacz-Bednarska A. Król T. Oncol. Clin. Pract. 2022;18:166–176.
-
- Cirri D. Chiaverini L. Pratesi A. Marzo T. Comments Inorg. Chem. 2023;43:465–478. doi: 10.1080/02603594.2022.2152016. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

