Role of PI3K/AKT/MAOA in glucocorticoid-induced oxidative stress and associated premature senescence of the trabecular meshwork
- PMID: 39688282
- PMCID: PMC11984687
- DOI: 10.1111/acel.14452
Role of PI3K/AKT/MAOA in glucocorticoid-induced oxidative stress and associated premature senescence of the trabecular meshwork
Abstract
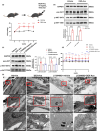
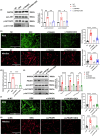
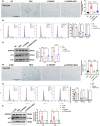
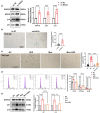
The oxidative stress-induced premature senescence of trabecular meshwork (TM) represents a pivotal risk factor for the development of glucocorticoid-induced glaucoma (GIG). This study aimed to elucidate the pathogenesis of TM senescence in GIG. MethodsIntraocular pressure (IOP), transmission electron microscopy and senescence-associated protein expression in TM were evaluated in GIG mice. Protein expression of phosphoinositide-3-kinase regulatory subunit 1 (PIK3R1) and monoamine oxidase A (MAOA), phosphorylation of AKT were quantified. ROS and mitochondrial superoxide levels were measured to evaluate cellular oxidative stress. Cell cycle analysis, β-galactosidase staining, senescence-associated protein expression were employed to assess the aging status of primary human trabecular meshwork cells (pHTMs). ResultsmRNA-seq and KEGG analysis indicating PI3K/AKT pathway as a key regulator in TM of GIG. PI3K inhibitor significantly prevented IOP elevation and abnormal mitochondrial morphology of TM in the GIG mouse model. PI3K inhibitor or selective silencing of PIK3R1 alleviated dexamethasone (DEX)-induced oxidative stress, also mitochondrial dysfunction, inhibiting MAOA expression in pHTMs. The same phenomenon was observed in the GIG models with inhibition of MAOA. Further KEGG analysis indicates that cellular senescence is the key factor in the pathogenesis of GIG. TM senescence was observed in both GIG mouse and cell models. Inhibition of the PI3K/AKT/MAOA pathway significantly alleviated DEX-induced premature cellular senescence of TM in GIG models. Glucocorticoids activated the PI3K/AKT/MAOA pathway, leading to mitochondrial dysfunction, oxidative stress, and premature aging in TM, elevating IOP. This mechanism could be associated with the onset and progression of GIG, providing a potential approach for its treatment.
Keywords: PI3K/AKT/MAOA; cell aging; glaucoma; glucocorticoid; mitochondria; oxidative stress; trabecular meshwork.
© 2024 The Author(s). Aging Cell published by Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that they do not have financial or other conflicts of interest.
Figures



References
-
- Antonucci, S. , Di Sante, M. , Tonolo, F. , Pontarollo, L. , Scalcon, V. , Alanova, P. , Menabo, R. , Carpi, A. , Bindoli, A. , Rigobello, M. P. , Giorgio, M. , Kaludercic, N. , & Di Lisa, F. (2021). The determining role of mitochondrial reactive oxygen species generation and monoamine oxidase activity in doxorubicin‐induced cardiotoxicity. Antioxidants & Redox Signaling, 34, 531–550. - PMC - PubMed
-
- Artzatbanov, V. , Muller, M. , & Azzi, A. (1987). Isolation and partial characterization of the cytochrome c oxidase of Micrococcus luteus (lysodeikticus). Archives of Biochemistry and Biophysics, 257, 476–480. - PubMed
-
- Caplan, A. , Fett, N. , Rosenbach, M. , Werth, V. P. , & Micheletti, R. G. (2017). Prevention and management of glucocorticoid‐induced side effects: A comprehensive review: A review of glucocorticoid pharmacology and bone health. Journal of the American Academy of Dermatology, 76, 1–9. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

