Alzheimer's Disease Risk Gene SORL1 Promotes Receptiveness of Human Microglia to Pro-Inflammatory Stimuli
- PMID: 39688327
- PMCID: PMC11845846
- DOI: 10.1002/glia.24659
Alzheimer's Disease Risk Gene SORL1 Promotes Receptiveness of Human Microglia to Pro-Inflammatory Stimuli
Abstract
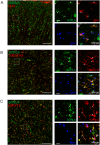
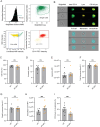
Sorting protein-related receptor containing class A repeats (SORLA) is an intracellular trafficking receptor encoded by the Alzheimer's disease (AD) gene SORL1 (sortilin-related receptor 1). Recent findings argue that altered expression in microglia may underlie the genome-wide risk of AD seen with some SORL1 gene variants, however, the functional significance of the receptor in microglia remains poorly explained. Using unbiased omics and targeted functional analyses in iPSC-based human microglia, we identified a crucial role for SORLA in sensitizing microglia to pro-inflammatory stimuli. We show that SORLA acts as a sorting factor for the pattern recognition receptor CD14, directing CD14 exposure on the cell surface and priming microglia to stimulation by pro-inflammatory factors. Loss of SORLA in gene-targeted microglia impairs proper CD14 sorting and blunts pro-inflammatory responses. Our studies indicate an important role for SORLA in shaping the inflammatory brain milieu, a biological process important to local immune responses in AD.
Keywords: Alzheimer's disease; SORLA; VPS10P domain receptors; brain inflammation; microglia.
© 2024 The Author(s). GLIA published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





References
-
- Andersen, M. N. , Al‐Karradi S. N. H., Kragstrup T. W., and Hokland M.. 2016. “Elimination of Erroneous Results in Flow Cytometry Caused by Antibody Binding to Fc Receptors on Human Monocytes and Macrophages.” Cytometry, Part A: Journal of the International Society for Analytical Cytology 89, no. 11: 1001–1009. 10.1002/cyto.a.22995. - DOI - PubMed
-
- Andersen, O. M. , Reiche J., Schmidt V., et al. 2005. “Neuronal Sorting Protein‐Related Receptor sorLA/LR11 Regulates Processing of the Amyloid Precursor Protein.” Proceedings of the National Academy of Sciences of the United States of America 102, no. 38: 13461–13466. 10.1073/pnas.0503689102. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

