Antiviral Use Among Children Hospitalized With Laboratory-Confirmed Influenza Illness: A Prospective, Multicenter Surveillance Study
- PMID: 39688383
- PMCID: PMC12497963
- DOI: 10.1093/cid/ciae573
Antiviral Use Among Children Hospitalized With Laboratory-Confirmed Influenza Illness: A Prospective, Multicenter Surveillance Study
Abstract
Background: Guidelines state that all hospitalized children with suspected or confirmed influenza receive prompt treatment with influenza-specific antivirals. We sought to determine the frequency of, and factors associated with, antiviral receipt among hospitalized children.
Methods: We conducted active surveillance of children presenting with fever or respiratory symptoms from 1 December 2016 to 31 March 2020 at 7 pediatric medical centers in the New Vaccine Surveillance Network. The cohort consisted of children hospitalized with influenza A or B confirmed by clinical or research testing. The primary outcome was frequency of antiviral receipt during hospitalization. We used logistic regression to obtain adjusted odds ratios (aORs) and 95% confidence intervals (CIs) for factors associated with antiviral receipt.
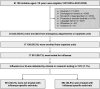
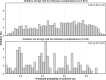
Results: A total of 1213 children with laboratory-confirmed influenza were included. Overall, 652 children (53.8%) received an antiviral. Roughly 63.0% of children received clinical influenza testing. Among those with clinical testing, 67.4% received an antiviral. Factors associated with higher odds of antiviral receipt included hematologic (aOR = 1.76; 95% CI = 1.03-3.02) or oncologic/immunocompromising (aOR = 2.41; 95% CI = 1.13-5.11) disorders, prehospitalization antiviral receipt (aOR = 2.34; 95% CI = 1.49-3.67), clinical influenza testing (aOR = 3.07; 95% CI = 2.28-4.14), and intensive care unit admission (aOR = 1.53; 95% CI = 1.02-2.29). Symptom duration >2 days was associated with lower odds of antiviral treatment (aOR = 0.40; 95% CI = .30-.52). Antiviral receipt varied by site with a 5-fold difference across sites.
Conclusions: Almost half of children hospitalized with influenza did not receive antivirals. Additional efforts to understand barriers to guideline adherence are crucial for optimizing care in children hospitalized with influenza.
Keywords: antivirals; guideline-concordant; influenza; oseltamivir; pediatrics.
© The Author(s) 2024. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. J. W. A. previously served as a member of the AstraZeneca Scientific Advisory Board. G. A. W. has received honoraria from Merck & Company for writing and revising chapters in the Merck Manual Electrophysiologic Studies. M. A. S. has received research funding from Pfizer and honoraria from Sanofi Pasteur for vaccine consultation. J. A. E. reports work as a consultant to AbbVie, AstraZeneca, Meissa Vaccines, Pfizer, Moderna, and Sanofi Pasteur and research to support their university from AstraZeneca, GSK, Merck, and Pfizer. M. G. M. reports research to support their university from Merk Sharpe & Dohme and a nonfinancial research grant from Viracor. F. M. M. reports work as a consultant for Moderna, GSK, AstraZeneca, Sanofi, Novavax, and Merck; serving on the data and safety monitoring boards for Pfizer, Moderna, the National Institutes of Health (NIH), Meissa, and Dynavax; and grant and research support from Pfizer, Gilead, NIH, and CDC. J. E. S. reports work as a consultant at the Association of American Medical Colleges. J. V. W. previously served as a member of the Quidel Scientific Advisory Board and on an independent data monitoring committee for GSK. S. M. O. reports travel support from the Gates Foundation. N. B. H. received grant support from Sanofi and Quidel, receives current grant support from Merck, and served on an advisory board for CSL Seqirus. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures


References
-
- Centers for Disease Control and Prevention . FluView interactive. Available at:https://www.cdc.gov/fluview/index.html. Accessed 19 December 2023.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

