Evaluation of the Accuracy of FreeStyle Libre 2 for Glucose Monitoring in White New Zealand Rabbits
- PMID: 39688518
- PMCID: PMC11651090
- DOI: 10.1002/vms3.70166
Evaluation of the Accuracy of FreeStyle Libre 2 for Glucose Monitoring in White New Zealand Rabbits
Abstract
Background: Studies are currently being conducted on rabbits requiring serial glucose monitoring. The FreeStyle Libre 2 (FSL2), a serial glucose monitoring device, has been validated in humans, dogs and cats, but not in rabbits.
Objectives: This study aimed to evaluate the accuracy of the FSL2 in rabbits.
Methods: Six healthy rabbits were used in this study. Interstitial glucose (IG) was measured using the FSL2, and blood glucose (BG) was measured using a portable blood glucose meter (PBGM); their results were compared with those from a clinical chemistry analyser. For the first 3 h, IG and BG were measured at 1-h intervals. Subsequently, they were measured every 8 h over a 48-h period. Regular insulin 0.2 U/kg was then administered to the rabbits, and IG and BG were measured every 15 min over a 90-min period.
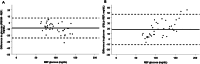
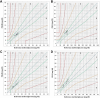
Results: Before insulin treatment, no measurements fell within the hypoglycaemic range (BG < 100 mg/dL). In the euglycaemic range (BG ≥ 100 mg/dL), the PBGM and FSL2 showed 85.7% and 23.8% accuracies, respectively. After insulin treatment, the PBGM showed 95.5% and 81.3% accuracies in the hypoglycaemic and euglycaemic ranges, respectively. The FSL2 showed 68.1% and 37.5% accuracies in the hypoglycaemic and euglycaemic ranges, respectively. Parkes consensus error grid analysis showed that the PBGM and FSL2 had 100% agreement for Zones A (no effect on clinical action) and B (altered clinical action unlikely to affect outcome) in rabbits with and without insulin treatment.
Conclusions: There was limited agreement between the FSL2 and reference standard BG measurements. However, the FSL2 allows clinically acceptable identification of hypoglycaemic states in rabbits.
Keywords: FSL2; FreeStyle Libre 2; hypoglycaemia; interstitial glucose; rabbit.
© 2024 The Author(s). Veterinary Medicine and Science published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
The FreeStyle Libre 2 system demonstrates limited analytical accuracy without resulting in detrimental clinical decisions.Am J Vet Res. 2025 Apr 25;86(8):ajvr.25.02.0055. doi: 10.2460/ajvr.25.02.0055. Print 2025 Aug 1. Am J Vet Res. 2025. PMID: 40280161
-
Interstitial glucose monitoring has acceptable clinical accuracy in juvenile dogs.J Am Vet Med Assoc. 2023 Jul 5;261(10):1475-1480. doi: 10.2460/javma.23.02.0103. Print 2023 Oct 1. J Am Vet Med Assoc. 2023. PMID: 37406997
-
Two human portable glucometers and a veterinary point-of-care glucometer correlate well with a reference laboratory chemistry analyzer for measurement of blood glucose concentrations in dogs.Am J Vet Res. 2025 Apr 2;86(6):ajvr.24.10.0317. doi: 10.2460/ajvr.24.10.0317. Print 2025 Jun 1. Am J Vet Res. 2025. PMID: 40179951
-
Continuous interstitial glucose monitoring in diabetic and non-diabetic critically ill patients is simple and accurate: comparison with venous, arterial and capillary glucose measurements.Acta Diabetol. 2025 Aug;62(8):1173-1181. doi: 10.1007/s00592-025-02531-1. Epub 2025 Jun 7. Acta Diabetol. 2025. PMID: 40481975 Free PMC article.
-
Pharmacological and electronic cigarette interventions for smoking cessation in adults: component network meta-analyses.Cochrane Database Syst Rev. 2023 Sep 12;9(9):CD015226. doi: 10.1002/14651858.CD015226.pub2. Cochrane Database Syst Rev. 2023. PMID: 37696529 Free PMC article.
References
-
- Cutler, D. C. , Koenig A., Di Girolamo N., and Mayer J.. 2020. “Investigation for Correction Formulas on the Basis of Packed Cell Volume for Blood Glucose Concentration Measurements Obtained With Portable Glucometers When Used in Rabbits.” American Journal of Veterinary Research 81, no. 8: 642–650. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

