Neuronal plasticity and its role in Alzheimer's disease and Parkinson's disease
- PMID: 39688547
- PMCID: PMC12094540
- DOI: 10.4103/NRR.NRR-D-24-01019
Neuronal plasticity and its role in Alzheimer's disease and Parkinson's disease
Abstract
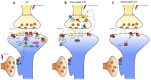
Neuronal plasticity, the brain's ability to adapt structurally and functionally, is essential for learning, memory, and recovery from injuries. In neurodegenerative diseases such as Alzheimer's disease and Parkinson's disease, this plasticity is disrupted, leading to cognitive and motor deficits. This review explores the mechanisms of neuronal plasticity and its effect on Alzheimer's disease and Parkinson's disease. Alzheimer's disease features amyloid-beta plaques and tau tangles that impair synaptic function, while Parkinson's disease involves the loss of dopaminergic neurons affecting motor control. Enhancing neuronal plasticity offers therapeutic potential for these diseases. A systematic literature review was conducted using databases such as PubMed, Scopus, and Google Scholar, focusing on studies of neuronal plasticity in Alzheimer's disease and Parkinson's disease. Data synthesis identified key themes such as synaptic mechanisms, neurogenesis, and therapeutic strategies, linking molecular insights to clinical applications. Results highlight that targeting synaptic plasticity mechanisms, such as long-term potentiation and long-term depression, shows promise. Neurotrophic factors, advanced imaging techniques, and molecular tools (e.g., clustered regularly interspaced short palindromic repeats and optogenetics) are crucial in understanding and enhancing plasticity. Current therapies, including dopamine replacement, deep brain stimulation, and lifestyle interventions, demonstrate the potential to alleviate symptoms and improve outcomes. In conclusion, enhancing neuronal plasticity through targeted therapies holds significant promise for treating neurodegenerative diseases. Future research should integrate multidisciplinary approaches to fully harness the therapeutic potential of neuronal plasticity in Alzheimer's disease and Parkinson's disease.
Keywords: Alzheimer’s disease; Parkinson’s disease; long-term depression; long-term potentiation; neuroinflammation; neuronal plasticity; synaptic plasticity.
Copyright © 2024 Neural Regeneration Research.
Conflict of interest statement
Figures


References
-
- Alkadhi KA. NMDA receptor-independent LTP in mammalian nervous system. Prog Neurobiol. 2021;200:101986. - PubMed
-
- Anwar MM. Oxidative stress-A direct bridge to central nervous system homeostatic dysfunction and Alzheimer’s disease. Cell Biochem Funct. 2022;40:17–27. - PubMed
-
- Anwar MM, Özkan E, Gürsoy-Özdemir Y. The role of extracellular matrix alterations in mediating astrocyte damage and pericyte dysfunction in Alzheimer’s disease: A comprehensive review. Eur J Neurosci. 2022;56:5453–5475. - PubMed
LinkOut - more resources
Full Text Sources

