Gut-brain axis and environmental factors in Parkinson's disease: bidirectional link between disease onset and progression
- PMID: 39688568
- PMCID: PMC11974660
- DOI: 10.4103/NRR.NRR-D-24-00994
Gut-brain axis and environmental factors in Parkinson's disease: bidirectional link between disease onset and progression
Abstract
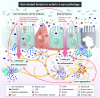
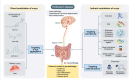
Parkinson's disease has long been considered a disorder that primarily affects the brain, as it is defined by the dopaminergic neurodegeneration in the substantia nigra and the brain accumulation of Lewy bodies containing α-synuclein protein. In recent decades, however, accumulating research has revealed that Parkinson's disease also involves the gut and uncovered an intimate and important bidirectional link between the brain and the gut, called the "gut-brain axis." Numerous clinical studies demonstrate that gut dysfunction frequently precedes motor symptoms in Parkinson's disease patients, with findings including impaired intestinal permeability, heightened inflammation, and distinct gut microbiome profiles and metabolites. Furthermore, α-synuclein deposition has been consistently observed in the gut of Parkinson's disease patients, suggesting a potential role in disease initiation. Importantly, individuals with vagotomy have a reduced Parkinson's disease risk. From these observations, researchers have hypothesized that α-synuclein accumulation may initiate in the gut and subsequently propagate to the central dopaminergic neurons through the gut-brain axis, leading to Parkinson's disease. This review comprehensively examines the gut's involvement in Parkinson's disease, focusing on the concept of a gut-origin for the disease. We also examine the interplay between altered gut-related factors and the accumulation of pathological α-synuclein in the gut of Parkinson's disease patients. Given the accessibility of the gut to both dietary and pharmacological interventions, targeting gut-localized α-synuclein represents a promising avenue for developing effective Parkinson's disease therapies.
Copyright © 2025 Neural Regeneration Research.
Conflict of interest statement
Figures
References
-
- Adams-Carr KL, Bestwick JP, Shribman S, Lees A, Schrag A, Noyce AJ. Constipation preceding Parkinson’s disease: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2016;87:710–716. - PubMed
-
- Amorim Neto DP, Bosque BP, Pereira de Godoy JV, Rodrigues PV, Meneses DD, Tostes K, Costa Tonoli CC, Faustino de Carvalho H, Gonzalez-Billault C, de Castro Fonseca M. Akkermansia muciniphila induces mitochondrial calcium overload and alpha-synuclein aggregation in an enteroendocrine cell line. iScience. 2022;25:103908. - PMC - PubMed
-
- Anderson C, Checkoway H, Franklin GM, Beresford S, Smith-Weller T, Swanson PD. Dietary factors in Parkinson’s disease: the role of food groups and specific foods. Mov Disord. 1999;14:21–27. - PubMed
LinkOut - more resources
Full Text Sources

