Respiratory syncytial virus infection in patients with haematological diseases: a retrospective multicentre study
- PMID: 39688808
- PMCID: PMC12316825
- DOI: 10.1007/s15010-024-02449-w
Respiratory syncytial virus infection in patients with haematological diseases: a retrospective multicentre study
Abstract
Purpose: This study aims to evaluate the burden of respiratory syncytial virus (RSV) infections in patients with haematological diseases. It seeks to analyse the relevance of prevention, diagnosis and treatment of RSV infections.
Methods: A multi-centre, retrospective study was conducted across University Hospitals in Cologne, Düsseldorf, Bonn, and the University Medical Centre Hamburg-Eppendorf between Jan 2016 and Aug 2023. All haematological patients with diagnosed RSV infection were included. The study focused on the incidence of RSV, underlying conditions, comorbidities, coinfections and clinical outcomes such as hospitalization, intensive care unit (ICU) admission and mortality.
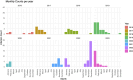
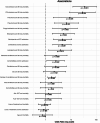
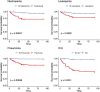
Results: Of 166 patients, 89 (53.6%) had signs of pneumonia and 37 (22.3%) were admitted to ICU due to RSV infection, while 20 (54%) of those were mechanically ventilated with median duration of 11 days (1,33; IQR:18). Mean age was 60 years (range 14-88). Sixteen patients (9.6%) were treated as outpatients, while 52 (31.3%) were hospitalized due to RSV infection; the median hospital stay was 16 days (IQR 25.25, range 0-97). 79 (47.6%) of patients presented with leukopenia and 57 (34.3%) with neutropenia. In total, 22 patients (13.3%) died within 30 days and 29 (17.5%) died within 90 days. Highest mortality rates were seen in patients with aggressive lymphoma (23.5%) and acute leukaemia (18%).
Conclusion: RSV significantly impacts patients with haematological diseases, leading to high rates of hospitalization, ICU admission, and mortality. Preventive measures, such as vaccination, alongside early diagnosis and individualized management, are essential to reduce RSV-associated morbidity and mortality in this high-risk population.
Keywords: Haematology; Infection prevention; RSV; Vaccine.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Competing interests: JS reports grants or contracts from the Federal Ministry of Education and Research Medical Faculty of the University of Cologne, from Basilea and Noscendo. Payments were made to his insti-tution. He received Consulting fees by Gilead, Mundipharma, Alvea Vax and Micron Research, payments or honoraria for presentations, speakers bureaus, manuscript writing or educational events by AbbVie, Hikma, Gilead, Pfizer. Receipt of equipment, materials, drugs, medical writing, gifts or other services payments made to the institution by Basilea.RM received travel support by Takeda and Janssen. She is part of the advisory board of Janssen.JSG is part of the Advisory board of Pfizer, not involved in the work presented, he received Speaker honoraria by AstraZeneca, Gilead Sciences, Menarini and Pfizer, not involved in the work presented.MBM received travel expenses and honoraria for lectures and/or consultancies from Abbvie, AstraZeneca, Gilead Sciences, Novavax, Pfizer, Takeda, ViiV Healthcare and Virology Edu-cation. FK declares that Patent applications on antibodies against viral pathogens have been filed by the University of Cologne, listing FK as inventor.OAC reports grants or contracts from BMBF, Cidara, DZIF, EU-DG RTD, F2G, Gilead, MedPace, MSD, Mundipharma, Octapharma, Pfizer, Scynexis; Consulting fees from Abbvie, AiCuris, Basilea, Biocon, Boston Strategic Partners, Cidara, Seqirus, Gilead, GSK, IQVIA, Janssen, Matinas, Med-Pace, Menarini, Molecular Partners, MSG-ERC, Mundipharma, Noxxon, Octapharma, Pardes, Part-ner Therapeutics, Pfizer, PSI, Scynexis, Seres, Shionogi, The Prime Meridian Group; Speaker and lecture honoraria from Abbott, Abbvie, Akademie für Infektionsmedizin, Al-Jazeera Pharmaceuti-cals/Hikma, amedes, AstraZeneca, Deutscher Ärzteverlag, Gilead, GSK, Grupo Biotoscana/United Medical/Knight, Ipsen Pharma, Medscape/WebMD, MedUpdate, MSD, Moderna, Mundipharma, Noscendo, Paul-Martini-Stiftung, Pfizer, Sandoz, Seqirus, Shionogi, streamedup!, Touch Inde-pendent, Vitis; Payment for expert testimony Cidara; Participation on a DRC, DSMB, Advisory Board for Cidara, IQVIA, Janssen, MedPace, PSI, Pulmocide, Vedanta Biosciences.SCM received research grants by DZIF (Deutsches Zentrum für Infektionsforschung) and the Uni-versity Hospital of Cologne. She received honoraria as a consultant by Octapharma and as a speaker by Pfizer. None of these involved in the work presented.SH, MC, PS, TH, HMO, CF, HG, UK, CL, JHN, TM declare no conflict of interests.
Figures
References
-
- 2. A. R. Falsey, P. A. Hennessey, M. A. Formica, C. Cox, und E. E. Walsh, „Respiratory syncytial virus infection in elderly and high-risk adults“, N Engl J Med, Bd. 352, Nr. 17, S. 1749–1759, Apr. 2005, doi: 10.1056/NEJMoa043951. - PubMed
-
- 4. D. Knipe u. a., Fields Virology, Volumes 1 and 2. Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical