Alpha-synuclein seed amplification assay longitudinal outcomes in Lewy body disease spectrum
- PMID: 39689039
- PMCID: PMC12129724
- DOI: 10.1093/brain/awae405
Alpha-synuclein seed amplification assay longitudinal outcomes in Lewy body disease spectrum
Abstract
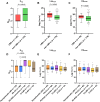
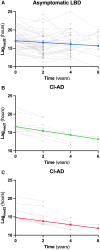
Evidence from neuropathological cohorts indicates that a CSF α-synuclein (α-syn) seed amplification assay (SAA) might provide quantitative kinetic parameters correlating with α-syn pathology burden in patients with Lewy body disease (LBD). Studies are needed to assess their longitudinal trend during the presymptomatic and clinical disease phases and their correlation with measures of disease progression. We aimed to assess the baseline α-syn CSF SAA kinetic parameters, their longitudinal variations and associations with clinical outcomes in a longitudinal cohort of repeatedly sampled LBD patients, including clinically unimpaired (asymptomatic LBD) and neurologically impaired individuals. Participants from the prospective BioFINDER-1 study with longitudinal CSF collections (n = 718) were screened by α-syn SAA. CSF samples were tested in four replicates blinded to clinical diagnoses. The number of positive replicates (Nrep), the time needed by the fluorescence signal to reach the threshold (Lag) and the highest intensity of the fluorescent signal were analysed at baseline (time of first positive SAA) in all participants and longitudinally in those with at least two α-syn-positive CSF samples available. One hundred and ninety-six individuals (whole cohort) showing α-syn seeding activity were included. Of those, 170 participants tested positive by SAA in all available samples, while 26 converted from a negative to a positive test result during follow-up (LBD-converters), suggesting an early LBD stage. At baseline, LBD-converters showed lower Nrep (P = 0.001) and a longer Lag (P = 0.001) than subjects displaying α-syn seeding activity from the first available sample. The Nrep increased longitudinally in the whole cohort [β = 0.09, 95% confidence interval (95% CI) 0.06-0.12, P < 0.001], in asymptomatic LBD (β = 0.15, 95% CI 0.09-0.21, P < 0.001) and in Parkinson's disease individuals without dementia (β = 0.07, 95% CI 0.02-0.12, P = 0.01). The Lag decreased longitudinally in asymptomatic LBD (β = -0.24, 95% CI -0.42 to -0.06, P = 0.008). Baseline Nrep predicted the subsequent appearance of dementia in the whole cohort [hazard ratio (HR) 1.57, 95% CI 1.19-2.07, P = 0.001] and the Parkinson's disease subgroup (HR 1.83, 95% CI 1.17-2.85, P = 0.008). The difference between the Lag at each sampling and that at baseline was negatively associated with the appearance of dementia in the whole cohort (HR 0.76, 95% CI 0.59-0.99, P = 0.04) and in the Parkinson's disease subgroup (HR 0.69, 95% CI 0.50-0.95, P = 0.02). The α-syn SAA parameters Nrep and Lag showed associations with the LBD stage and the development of dementia. Furthermore, their longitudinal variation is coherent with progression of pathology over time. These data support the use of SAA kinetic parameters to monitor disease progression and therapeutic response.
Keywords: Lewy body disease; disease progression; kinetic parameters; longitudinal study; prion; seed amplification assay.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
O.H. has acquired research support (for the institution) from AVID Radiopharmaceuticals, Biogen, C2N Diagnostics, Eli Lilly, Eisai, Fujirebio, GE Healthcare and Roche. In the past 2 years, he has received consultancy/speaker fees from AC Immune, Alzpath, BioArctic, Biogen, Bristol Meyer Squibb, Cerveau, Eisai, Eli Lilly, Fujirebio, Merck, Novartis, Novo Nordisk, Roche, Sanofi and Siemens. S.P. has acquired research support (for the institution) from ki elements/ADDF and Avid. In the past 2 years, he has received consultancy/speaker fees from Bioartic, Biogen, Esai, Lilly and Roche. None of the other authors has any disclosures.
Figures
References
-
- Höglinger GU, Adler CH, Berg D, et al. A biological classification of Parkinson’s disease: The SynNeurGe research diagnostic criteria. Lancet Neurol. 2024;23:191–204. - PubMed
-
- Simuni T, Chahine LM, Poston K, et al. A biological definition of neuronal α-synuclein disease: Towards an integrated staging system for research. Lancet Neurol. 2024;23:178–190. - PubMed
MeSH terms
Substances
Grants and funding
- Ricerca Corrente
- Ministero della Salute
- #NextGenerationEU
- Ministry of University and Research
- PE0000006/National Recovery and Resilience Plan
- ADG-101096455/ERC_/European Research Council/International
- ZEN24-1069572/ALZ/Alzheimer's Association/United States
- SG-23-1061717/ALZ/Alzheimer's Association/United States
- GHR Foundation
- 2021-02219/Swedish Research Council
- 2022-00775/Swedish Research Council
- 2022-0231/Knut and Alice Wallenberg Foundation
- Strategic Research Area MultiPark
- AF-980907/Swedish Alzheimer Foundation
- AF-994229/Swedish Alzheimer Foundation
- FO2021-0293/Swedish Brain Foundation
- FO2023-0163/Swedish Brain Foundation
- 1412/22/Parkinson foundation of Sweden
- Cure Alzheimer's fund
- WASP/DDLS22-066/Rönström Family Foundation
- 2020-O000028/Skåne University Hospital Foundation
- 2022-1259/Regionalt Forskningsstöd
- 2022-Projekt0080/Regionalt Forskningsstöd
- 2022-Projekt0107/Regionalt Forskningsstöd
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

