Structure-based virtual screening of Trachyspermum ammi metabolites targeting acetylcholinesterase for Alzheimer's disease treatment
- PMID: 39689077
- PMCID: PMC11651584
- DOI: 10.1371/journal.pone.0311401
Structure-based virtual screening of Trachyspermum ammi metabolites targeting acetylcholinesterase for Alzheimer's disease treatment
Retraction in
-
Retraction: Structure-based virtual screening of Trachyspermum ammi metabolites targeting acetylcholinesterase for Alzheimer's disease treatment.PLoS One. 2025 May 8;20(5):e0324094. doi: 10.1371/journal.pone.0324094. eCollection 2025. PLoS One. 2025. PMID: 40338826 Free PMC article. No abstract available.
Abstract
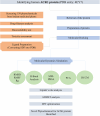
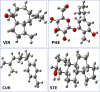
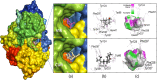
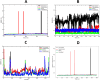
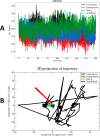
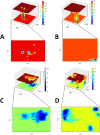
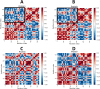
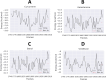
In recent decades, Alzheimer's disease (AD) has garnered significant attention due to its rapid global prevalence. The cholinergic hypothesis posits that the degradation of acetylcholine by acetylcholinesterase (AChE) contributes to AD development. Despite existing anti-AChE drugs, their adverse side effects necessitate new agents. This study analyzed 150 bioactive phytochemicals from Trachyspermum ammi using structure-based drug design and various in-silico tools to identify potent anti-AChE compounds. Compounds were screened for drug-likeness (QEDw ≥50%) and bioavailability (≥55%) and underwent toxicity profiling via the ProTox-II server. Selected compounds were prepared for molecular docking with the human AChE protein as the receptor. Viridifloral, 2-Methyl-3-glucosyloxy-5-isopropyl phenol, Alpha-Curcumene, and Sterol emerged as top candidates with high AChE affinity. These results were validated by molecular dynamics simulations, confirming stable interactions. The hit compounds were further evaluated for drug-likeness using Lipinski's rule and ADMET properties, confirming favorable pharmacokinetic profiles. DFT optimization analyzed frontier molecular orbitals and electrostatic potential, demonstrating favorable chemical reactivity and stability. This study suggests that these identified compounds could be novel nature-derived AChE inhibitors, potentially contributing to AD treatment. However, further in-vitro and in-vivo studies are necessary to confirm their efficacy in biological systems. Future research will focus on developing these compounds into safe and effective drugs to combat Alzheimer's disease.
Copyright: © 2024 Musa et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- World Health Organization. Fact sheets: dementia [Internet]. Available from: https://www.who.int/news
-
- Gauthier S, Feldman HH, Schneider LS, Wilcock GK, Frisoni GB, Hardlund JH, et al. Efficacy and safety of tau-aggregation inhibitor therapy in patients with mild or moderate Alzheimer’s disease: a randomised, controlled, double-blind, parallel-arm, phase 3 trial. Lancet. 2016. Dec 10;388(10062):2873–2884. doi: 10.1016/S0140-6736(16)31275-2 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

