Development of a new methodology for the determination of PET microplastics in sediment, based on microwave-assisted acid digestion
- PMID: 39689124
- PMCID: PMC11651601
- DOI: 10.1371/journal.pone.0314520
Development of a new methodology for the determination of PET microplastics in sediment, based on microwave-assisted acid digestion
Abstract
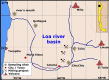
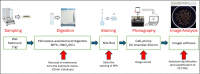
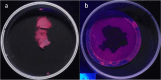
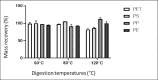
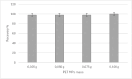
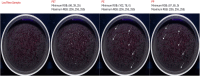
Analytical methods for the determination of microplastics in sediments typically involve matrix drying, sieving, grinding, and flotation as part of the sample treatment. However, the real need for these steps and analytical validation studies are scarce. This work proposes a method that avoids the drying, sieving, and flotation procedures by using a direct acid attack of HNO₃/HCl (3:1) on wet sediment samples, assisted by microwave digestion. For detection, induced fluorescence using a UV camera, with Nile Red (NR) as the fluorophore and a cell phone camera for image capture were used. The results showed that when the digestion temperature was raised to 120°C, PET recovery decreased due to plastic particle fusion. However, at 60°C, microwave digestion resulted in a 97% recovery of PET particles, eliminating chitin interference and canceling cellulose fluorescence without the need for flotation. This method proved effective for monitoring plastic microparticles in sediments from the Loa River, Chile, revealing that the river is predominantly contaminated with PET microparticles, particularly upstream in the Taira area.
Copyright: © 2024 Perez et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures






References
-
- Lu HC, Ziajahromi S, Neale PA, Leusch FDL. A systematic review of freshwater microplastics in water and sediments: Recommendations for harmonisation to enhance future study comparisons. Science of the Total Environment. 2021;781. doi: 10.1016/j.scitotenv.2021.146693 - DOI
-
- Phuong NN, Fauvelle V, Grenz C, Ourgaud M, Schmidt N, Strady E, et al.. Highlights from a review of microplastics in marine sediments. Science of the Total Environment. 2021;777. doi: 10.1016/j.scitotenv.2021.146225 - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

