Enhanced detection of distinct honeycomb-structured neuronal SMARCC2 cytobodies in Parkinson's Disease via Cyclic Heat-Induced Epitope Retrieval (CHIER)
- PMID: 39689145
- PMCID: PMC11651576
- DOI: 10.1371/journal.pone.0315183
Enhanced detection of distinct honeycomb-structured neuronal SMARCC2 cytobodies in Parkinson's Disease via Cyclic Heat-Induced Epitope Retrieval (CHIER)
Abstract
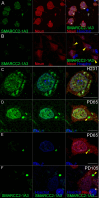
Antigen retrieval is crucial for immunohistochemistry, particularly in formalin-fixed paraffin-embedded brain tissue, where fixation causes extensive crosslinking that masks epitopes. Heat Induced Epitope Retrieval (HIER) reverses these crosslinks, improving access to nuclear and aggregated proteins. We introduce Cyclic Heat-Induced Epitope Retrieval (CHIER), an advanced technique that builds on HIER by incorporating repeated cycles of heating and cooling. CHIER optimises antigen retrieval and significantly improves detection. CHIER is particularly effective for detecting chromatin-binding proteins, such as SMARCC2, which are difficult to label using conventional IHC methods. Using CHIER on formalin-fixed paraffin-embedded human brain sections, we achieved robust detection of SMARCC2 in both the nucleus and cytoplasm. CHIER also enhanced the visualisation of large SMARCC2+ cytoplasmic bodies, termed cytobodies, which are increased in Parkinson's Disease (PD). Our findings suggest that SMARCC2 may translocate from the nucleus to the cytoplasm in PD, potentially implicating SMARCC2 aggregation in the disease's pathology. Furthermore, CHIER does not negatively impact the antigenicity of other antibodies, supporting its use for multiplex fluorescent immunohistochemistry and super-resolution imaging. These results highlight CHIER's potential for improving the detection of chromatin-binding and aggregated proteins in neurodegenerative disease research, offering new insights into SMARCC2's role in Parkinson's Disease.
Copyright: © 2024 Carmichael-Lowe et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




Similar articles
-
A single simple procedure for dewaxing, hydration and heat-induced epitope retrieval (HIER) for immunohistochemistry in formalin fixed paraffin-embedded tissue.Eur J Histochem. 2015 Nov 3;59(4):2532. doi: 10.4081/ejh.2015.2532. Eur J Histochem. 2015. PMID: 26708177 Free PMC article.
-
Selection of buffer pH by the isoelectric point of the antigen for the efficient heat-induced epitope retrieval: re-appraisal for nuclear protein pathobiology.Histochem Cell Biol. 2009 Dec;132(6):659-67. doi: 10.1007/s00418-009-0635-8. Epub 2009 Sep 19. Histochem Cell Biol. 2009. PMID: 19768463
-
Superior performance of decloaking chamber-based heat-induced epitope retrieval method improves the quantification of Olig2 cells in paraffin-embedded section of embryonic mouse brain.J Neurosci Methods. 2014 Sep 30;235:226-33. doi: 10.1016/j.jneumeth.2014.06.032. Epub 2014 Jul 11. J Neurosci Methods. 2014. PMID: 25020254
-
[Antigen retrieval: its significance and drawbacks in immunohistochemistry].Kaibogaku Zasshi. 1996 Dec;71(6):615-28. Kaibogaku Zasshi. 1996. PMID: 9038004 Review. Japanese.
-
An inflammatory review of Parkinson's disease.Prog Neurobiol. 2002 Dec;68(5):325-40. doi: 10.1016/s0301-0082(02)00127-2. Prog Neurobiol. 2002. PMID: 12531233 Review.
References
-
- Wiseman JA, Murray HC, Faull RLMF, Dragunow M, Turner CP, Dieriks BV, et al.. Aggregate-prone brain regions in Parkinson’s disease are rich in unique N-terminus α-synuclein conformers with high proteolysis susceptibility. Npj Park Dis. 2024;10: 1–18. doi: 10.1038/s41531-023-00614-w - DOI - PMC - PubMed
-
- Kitamoto T, Ogomori K, Tateishi J, Prusiner S. Formic acid pretreatment enhances immunostaining of cerebral and systemic amyloids. Lab Investig J Tech Methods Pathol. 1987;57: 230–236. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

