Castration-resistant prostate cancer is resensitized to androgen deprivation by autophagy-dependent apoptosis induced by blocking SKP2
- PMID: 39689183
- PMCID: PMC11784317
- DOI: 10.1126/scisignal.adk4122
Castration-resistant prostate cancer is resensitized to androgen deprivation by autophagy-dependent apoptosis induced by blocking SKP2
Abstract
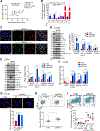
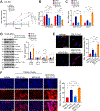
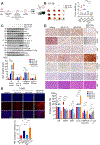
Resistance to androgen receptor (AR)-targeted therapies for prostate cancer (PCa) is characteristic of an aggressive subtype called castration-resistant prostate cancer (CRPC) and is often associated with tumor relapse. Both relapse and poor prognosis in patients with CRPC are associated with increased abundance of the E3 ubiquitin ligase SKP2. Therefore, we investigated the therapeutic potential of combined inhibition of AR and SKP2 for CRPC. We found that combined targeting of AR and SKP2 with small-molecule inhibitors decreased proliferation in two CRPC cell lines in culture and in xenografts in humanized mice. Furthermore, combined therapy in mice markedly decreased the growth of Pten/Trp53 double-knockout tumors, a particularly invasive model of CRPC, whereas disruption of either AR or SKP2 alone only modestly suppressed their growth. Mechanistically, the inhibition of SKP2 in CRPC cells induced autophagy-dependent apoptosis and promoted luminal-associated phenotypes, which promoted responsiveness to AR-targeted therapy. These effects were further enhanced by coinhibition of AR and were not induced by the AR inhibitor alone. Our findings indicate that targeting both AR and SKP2 signaling pathways is necessary to treat CRPC.
Conflict of interest statement
Figures

References
-
- Siegel RL, Miller KD, Wagle NS, Jemal A, Cancer statistics, 2023. CA Cancer J Clin 73, 17–48 (2023). - PubMed
-
- Sung H et al., Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 71, 209–249 (2021). - PubMed
-
- Isaacs JT, Isaacs WB, Androgen receptor outwits prostate cancer drugs. Nat Med 10, 26–27 (2004). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

